ID: PMRREP33054| 193 Pages | 21 Jan 2025 | Format: PDF, Excel, PPT* | Healthcare

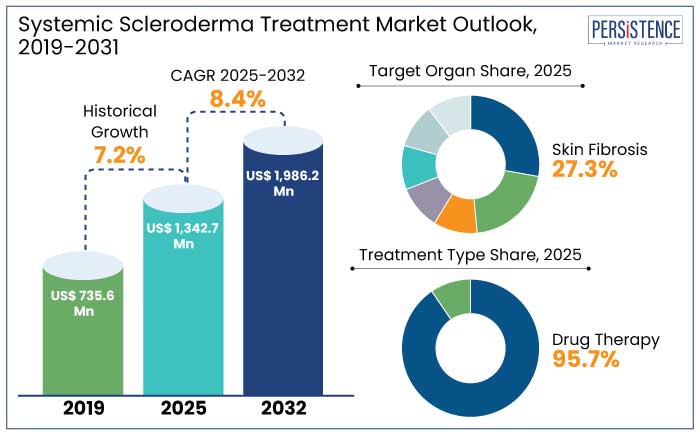
The systemic scleroderma treatment market is projected to witness a CAGR of 8.4% during the forecast period from 2025 to 2032. It is anticipated to increase from US$ 1342.7 Mn recorded in 2025 to US$ 1986.2 Mn by 2032.
The systemic scleroderma treatment market is witnessing robust growth, driven by advancements in therapeutics and an increasing focus on improving patient outcomes for this rare autoimmune disease.
Systemic scleroderma, characterized by skin thickening and internal organ involvement, poses significant treatment challenges due to its complex pathology. Recent developments in targeted biologics and immunomodulatory therapies are transforming the treatment landscape, offering new hope to patients.
Drugs such as interleukin inhibitors, endothelin receptor antagonists, and tyrosine kinase inhibitors are gaining traction for their potential to address both fibrotic and inflammatory pathways associated with systematic scleroderma. One of the key drivers for the market is the increasing prevalence of autoimmune diseases globally.
The increasing awareness among healthcare providers about early diagnosis and treatment is fostering demand for advanced therapies. Regulatory approvals for novel drugs and expanded indications for existing treatments further support systemic scleroderma treatment market growth. For instance,
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Global Systemic Scleroderma Treatment Market Size (2025E) |
US$ 1342.7 Mn |
|
Projected Market Value (2032F) |
US$ 1986.2 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
8.4% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
7.2% |
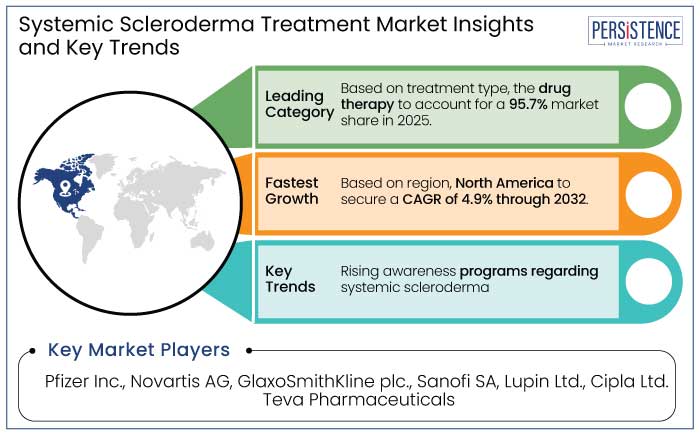
North America leads the systemic scleroderma treatment market with a 47.1% market share in 2025 due to a combination of robust healthcare infrastructure, extensive research initiatives, and a high prevalence of the disease. The region’s advanced diagnostic capabilities enable early detection, which is critical for managing a condition as complex as systematic scleroderma. North America is estimated to showcase a CAGR of 4.9% through 2032.
According to the Scleroderma Foundation, approximately 300,000 individuals in the U.S. are affected by scleroderma, of which one-third have the systemic form. This significant patient pool drives demand for effective treatment options.
North America is home to leading pharmaceutical and biotechnology companies that invest heavily in research and development. These companies are at the forefront of introducing innovative therapies, such as immunomodulators and biologics, targeting systemic scleroderma's underlying mechanisms. Regulatory support from agencies like the FDA further facilitates the approval and availability of novel treatments.
Skin fibrosis is the hallmark manifestation of systemic scleroderma, making it the leading target in the treatment market and to account for a 27.3% market share in 2025. This condition arises from excessive collagen deposition, which leads to skin thickening, stiffness, and impaired mobility.
Skin fibrosis significantly impacts patients' quality of life, often causing pain, reduced dexterity, and disfigurement as the most visible and debilitating feature of the disease. Moreover, its progression serves as a clinical marker for disease severity and can indicate systemic involvement, including pulmonary and renal complications.
The treatment market prioritizes skin fibrosis due to its complex pathophysiology, which involves immune dysregulation, fibroblast activation, and vascular abnormalities. Therapeutic advancements are focused on targeting fibrotic pathways to halt or reverse collagen buildup.
The visibility of skin fibrosis makes it a key metric for evaluating treatment efficacy during clinical trials, further driving its focus. Innovations such as antifibrotic drugs, monoclonal antibodies, and combination therapies aim to address this pressing unmet need.
The injectable route of administration is a leader in the systemic scleroderma treatment market and to account for a 49.1% market share in 2025. It is due to its unmatched efficacy and rapid therapeutic action. Systemic scleroderma, a chronic autoimmune disorder, often requires targeted and prompt delivery of medications to address widespread inflammation, fibrosis, and vascular complications.
Injectable therapies such as intravenous prostacyclin analogs and immunosuppressive agents, ensure accurate dosing with optimal bioavailability compared to oral or topical alternatives, which may face barriers like gastrointestinal degradation or poor absorption.
Injectables enable the administration of biologics, which have revolutionized scleroderma treatment by targeting specific immune pathways, such as IL-6 inhibitors or anti-fibrotic agents. These biologics often require precise delivery through injection to maintain their molecular stability and efficacy.
Many patients are not able to understand the functioning and overall perspective of systemic scleroderma. Many demographic parameters influence the scenario, and every patient and healthcare person has a different point of view and needs regarding systemic scleroderma disease.
In the study by the National Library of Medicine, a survey of 242 systemic scleroderma patients was conducted to gain information about their unmet needs. Out of 91 questions, 9 provided demographic data, whereas 72 questions addressed daily living, psychological, physical, spiritual, existential, health services, health information, social support, and employment issues.
It was found that the most prevalent unmet needs in systemic scleroderma patients included existential domain/psychologic, fear about the disease, anxiety and stress, depression, and many more adverse conditions.
Unmet medical needs need to be addressed time by time, and many programs and surveys globally have been introduced to understand the unmet needs of the patients suffering from such rare diseases.
The global systemic scleroderma treatment market recorded a CAGR of 7.2% during the period from 2019 to 2023. The market witnessed steady growth over the past decade, driven by advancements in immunology, the introduction of targeted biologics, and increasing disease awareness.
Treatment options were limited to off-label use of immunosuppressants and anti-inflammatory drugs, focusing primarily on symptom management rather than disease modification. However, the launch of novel therapies, such as IL-6 inhibitors and endothelin receptor antagonists, has transformed the treatment landscape.
The market fueled by rising diagnostic rates, increasing healthcare expenditure, and enhanced availability of advanced therapeutics in developed regions. The trend toward personalized medicine and biologic-based treatments further contributed to market expansion.
The forecast period indicates accelerated growth, supported by ongoing research into disease-modifying therapies and the expansion of treatment access in emerging markets. Innovations in drug delivery systems, such as sustained release injectables and oral formulations, are expected to enhance patient adherence.
The approval pipeline for therapies targeting fibrosis and vascular complications is robust, promising significant breakthroughs. These factors, coupled with a growing patient population, are poised to drive substantial market expansion in the coming years.
Increasing Prevalence of Systemic Scleroderma
The increasing prevalence of systemic scleroderma is a critical driver of the systemic scleroderma treatment market. While the condition remains rare, affecting approximately 10-20 individuals per million annually, recent studies indicate a gradual increase in incidence rates globally.
Advances in early detection are playing a pivotal role. Systemic scleroderma was often diagnosed at advanced stages due to its heterogeneous presentation. However, the growing use of autoantibody profiling, such as anti-topoisomerase and anti-centromere antibodies, has improved early-stage diagnosis. This trend has resulted in early initiation of treatment, driving market further for disease-modifying drugs and immunosuppressants that can delay disease progression.
High Treatment Costs
The high treatment costs for systemic scleroderma present a significant barrier to effective management, especially in low-income regions and developing countries. Treatments for systemic scleroderma often involve biological drugs, immunosuppressants, and other specialized therapies, many of which are priced at premium levels.
Biologic drugs, such as tocilizumab and rituximab, while effective in managing symptoms and slowing disease progression, can cost thousands of dollars per month. Immunosuppressants like methotrexate and cyclophosphamide are also costly, and their long-term use can further increase the financial burden on patients.
Patients may need frequent hospital visits, laboratory tests, and physical therapy in addition to the direct costs of medications, further compounding the financial strain. This financial barrier disproportionately affects patients in low- and middle-income countries, where healthcare systems may not be well-equipped to provide access to such expensive treatments.
Patients often rely on generic medications in these regions which may be less effective or not well-suited to their specific needs, leading to suboptimal treatment outcomes. Even in high-income countries, insurance coverage for systemic scleroderma treatments can be inconsistent. As a result, many patients may delay or forgo necessary treatment, worsening their condition and potentially leading to complications that require even expensive interventions.
The financial strain associated with these treatments can lead to psychological stress, which may further impact the patient health. The combination of high treatment costs, inadequate healthcare infrastructure in some regions, and the need for long-term management of a chronic condition creates a significant barrier to effective systemic scleroderma treatment.
Biologic Therapies for Fibrosis
Biologic therapies targeting fibrosis hold immense promise for revolutionizing the treatment landscape of systemic scleroderma, a condition marked by progressive tissue fibrosis that compromises multiple organ systems, including the skin, lungs, and gastrointestinal tract.
The central pathological feature of systemic scleroderma is the excessive accumulation of extracellular matrix components, primarily collagen, due to the over activation of fibrotic pathways.
The transforming growth factor-beta (TGF-β) signaling pathway is a critical driver of fibrosis, making it a prime target for therapeutic intervention. TGF-β plays a pivotal role in the initiation and progression of fibrosis by stimulating fibroblast activation, collagen deposition, and the transformation of normal tissue into scar tissue.
Inhibiting TGF-β signaling has emerged as a potential strategy for preventing or reversing the fibrotic process in systemic scleroderma. Several biological therapies, such as monoclonal antibodies and small molecule inhibitors, are currently under investigation to block TGF-β or other key molecules involved in fibrotic signaling. For instance,
Competition is intensifying with the rise of biosimilars and alternative treatment modalities, such as stem cell therapies and regenerative medicine. Additionally, geographic expansion strategies by leading players into underserved markets, particularly in Asia Pacific and Latin America, are broadening patient access.
Significant unmet needs in disease modification and holistic care present opportunities for new entrants to disrupt the market despite the competitive dynamics ensuring continuous innovation in this challenging therapeutic area.
Recent Industry Developments
Yes, the market is set to reach US$ 1342.7 Mn in 2025.
North America is expected to witness a high market share.
A few of the leading market players in the market are Pfizer Inc. and Novartis AG.
The market is estimated to report a CAGR of 8.4% through 2031.
Increasing prevalence of systemic scleroderma remains a key driver for market growth.
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Target Organ
By Treatment Type
By Route of Administration
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author