ID: PMRREP19603| 211 Pages | 22 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

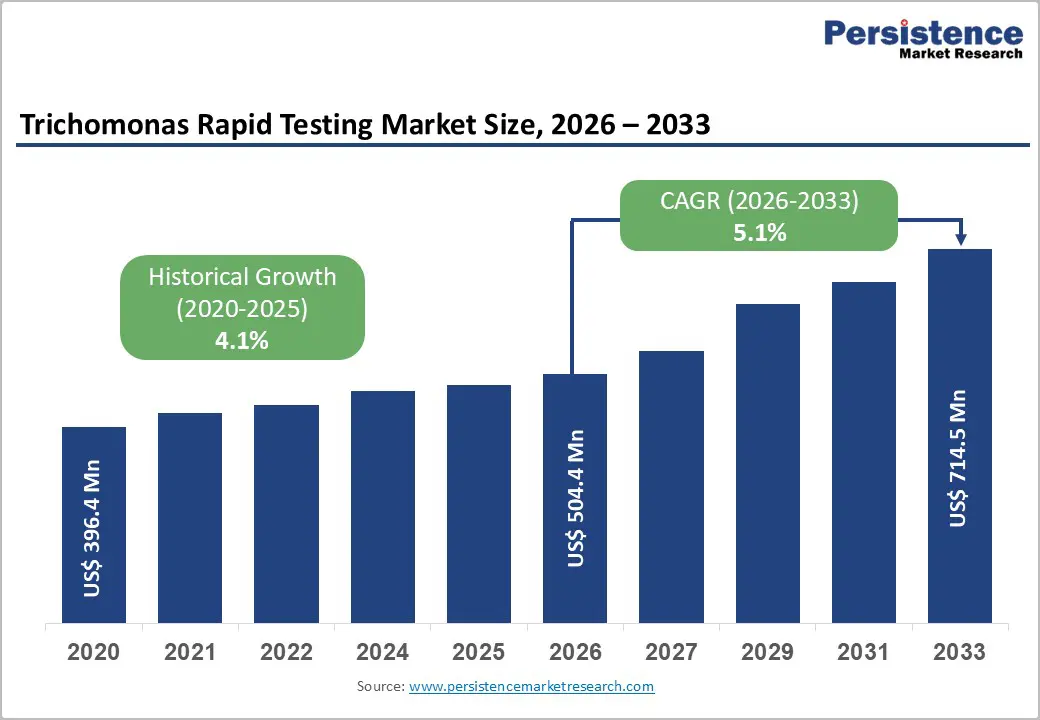
The global trichomonas rapid testing market size is expected to be valued at US$ 504.4 million in 2026 and projected to reach US$ 714.5 million by 2033, growing at a CAGR of 5.1% between 2026 and 2033.
The Trichomonas rapid testing market is expanding due to the rising global burden of trichomoniasis, with the World Health Organization reporting 156 million new cases in 2020 among individuals aged 15 49. This high prevalence highlights the need for early and accessible diagnosis.
Advancements in point-of-care rapid tests improve screening efficiency, especially in low-resource settings. Growing awareness of sexually transmitted infections (STIs) and routine STI screening initiatives further support demand across hospitals, clinics, and public health programs.
| Key Insights | Details |
|---|---|
| Trichomonas Rapid Testing Market Size (2026E) | US$ 504.4 Mn |
| Market Value Forecast (2033F) | US$ 714.5 Mn |
| Projected Growth (CAGR 2026 to 2033) | 5.1% |
| Historical Market Growth (CAGR 2020 to 2025) | 4.1% |
The increasing global prevalence of sexually transmitted infections significantly drives growth of the Trichomonas rapid testing market. According to the World Health Organization, nearly 374 million new cases of curable STIs including chlamydia, gonorrhea, syphilis, and trichomoniasis are reported annually, with trichomoniasis alone accounting for about 156 million cases worldwide in 2020. A large proportion of infections remain asymptomatic, especially in women, increasing the risk of complications such as pelvic inflammatory disease, infertility, and adverse pregnancy outcomes. This rising disease burden has intensified the need for early detection and timely treatment. As a result, healthcare systems increasingly rely on rapid diagnostic tests to enable prompt diagnosis, reduce transmission, and support global STI control initiatives.
The transition from laboratory-based diagnostics to point-of-care testing (POCT) is a major growth driver for the Trichomonas rapid testing market. POCT solutions offer fast turnaround times, often delivering results within 10 minutes, enabling same-visit diagnosis and treatment. Many antigen-based rapid tests demonstrate high performance, with sensitivity around 96% and specificity near 95% for vaginal swab samples. These advantages reduce patient follow-up loss, lower healthcare costs, and improve treatment adherence. POCT is particularly valuable in primary care clinics, sexual health centers, and resource-limited settings where laboratory infrastructure is inadequate. Increasing integration of rapid tests into routine STI screening programs further enhances accessibility and supports sustained market expansion globally.
Stringent regulatory frameworks present a significant restraint for the Trichomonas rapid testing market. In developed regions, regulatory authorities require extensive clinical validation and performance data before approving new diagnostic products. In the United States, FDA clearance for in vitro diagnostic tests involves complex and time-consuming approval pathways, while compliance with Regulation (EU) 2017/746 in Europe further increases regulatory burden. These processes extend product development timelines and raise associated costs, particularly impacting small and mid-sized manufacturers. Differences in regulatory requirements across regions also complicate international market entry and product commercialization. As a result, innovation cycles slow down, limiting the availability of novel rapid testing technologies despite rising clinical demand.
Cost sensitivity remains a major challenge in emerging and low-income markets, restricting the adoption of advanced Trichomonas rapid testing solutions. In regions such as Latin America, Africa, and parts of the Middle East, constrained healthcare budgets often favor low-cost diagnostic methods, including wet-mount microscopy, despite their lower sensitivity. Advanced rapid tests, particularly molecular-based devices, carry higher upfront costs that limit affordability in public healthcare systems. Lack of reimbursement coverage and limited awareness further reduce adoption. Consequently, although disease prevalence is high in these regions, penetration of premium rapid testing kits remains limited, slowing overall market growth and widening diagnostic gaps.
Technological advancements in molecular diagnostics present a strong growth opportunity for the Trichomonas rapid testing market. Innovations such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) based devices offer superior diagnostic accuracy, with reported sensitivities ranging from 98.6% to nearly 100%. These tests significantly outperform traditional microscopy and antigen-based methods, making them ideal for high-volume laboratories, reference centers, and tertiary care hospitals. Improved accuracy supports early detection, reduces false negatives, and enhances patient outcomes, particularly in high-risk populations.
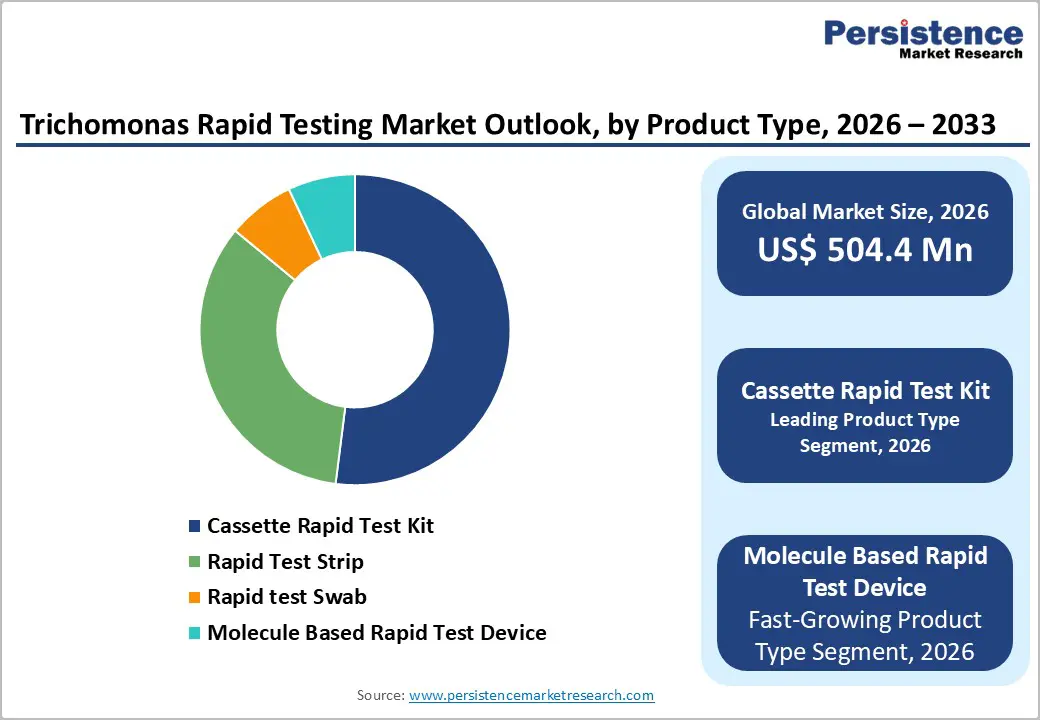
The ongoing evolution of point-of-care molecular platforms further strengthens this opportunity. Miniaturized, portable molecular devices are bridging the gap between laboratory accuracy and rapid testing convenience. At the same time, manufacturers focusing on rapid test strips identified as the fastest-growing product segment can leverage innovations in assay design to improve sensitivity while maintaining affordability and ease of use. As global STI screening initiatives expand and demand for reliable diagnostics increases, technologically advanced molecular and rapid test solutions are well positioned to capture future market growth.
Cassette Rapid Test Kits are expected to lead the Trichomonas Rapid Testing Market with an estimated 52% market share in 2025, driven by their operational simplicity, affordability, and broad applicability across diverse healthcare settings. These kits are widely adopted in clinics, hospitals, community health centers, and outreach programs due to their ease of use and minimal training requirements. Cassette-based tests deliver results within minutes while maintaining high sensitivity and specificity, making them well suited for point-of-care testing (POCT). Their compatibility with decentralized testing environments supports early diagnosis and same-visit treatment, which is critical for controlling transmission of trichomoniasis. Additionally, preference for antigen-based detection methods in routine STI screening programs strengthens adoption. Compared to molecular-based devices, cassette kits offer a cost-effective balance between accuracy and accessibility, reinforcing their dominance over more complex and infrastructure-dependent alternatives in both developed and emerging markets.
Hospitals are projected to account for approximately 45% of the Trichomonas Rapid Testing Market share in 2025, supported by high patient inflow and structured STI screening protocols. These facilities routinely integrate rapid testing into emergency departments, gynecology units, and infectious disease clinics, enabling timely diagnosis and treatment. Hospitals benefit from advanced infrastructure, trained personnel, and access to multiple diagnostic modalities, facilitating large-scale deployment of rapid test kits. Routine and symptom-based STI screenings contribute to higher detection rates, particularly in high-risk populations. The ability to deliver immediate results supports prompt clinical decision-making, reduces loss to follow-up, and improves patient outcomes. Furthermore, hospital-based testing aligns with national and international STI management guidelines, reinforcing consistent demand. As awareness of asymptomatic trichomoniasis increases, hospitals continue to serve as primary testing hubs, maintaining their dominant position among end users.
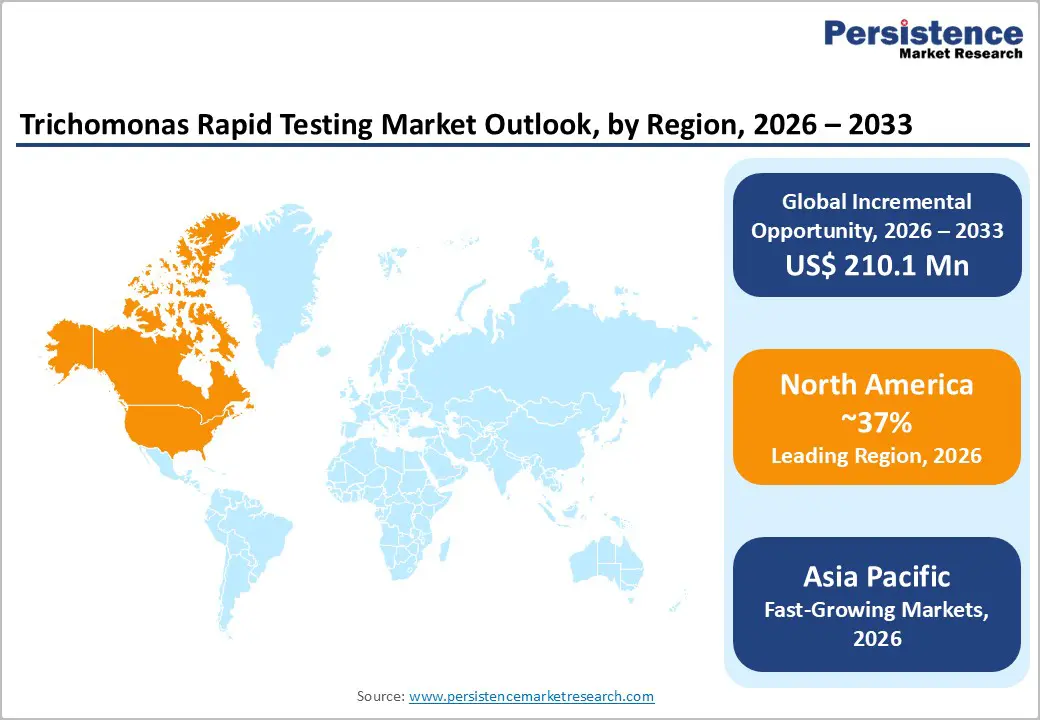
North America accounted for approximately 37.0% of the global Trichomonas Rapid Testing Market share in 2025, driven by high disease awareness, established screening programs, and strong healthcare infrastructure. In the United States, Trichomonas vaginalis is the most common non-viral sexually transmitted infection. According to the Centers for Disease Control and Prevention (CDC) data from 2021, the prevalence of T. vaginalis is estimated at 2.1% in females and 0.5% in males, with significantly higher rates among Black females (9.6%) and Black males (3.6%) compared to non-Hispanic White and Hispanic populations. Unlike chlamydia and gonorrhea, trichomoniasis prevalence remains similar among women above and below 24 years of age, supporting broader screening needs. Rising infection rates, coupled with CDC-backed testing recommendations, are increasing awareness and adoption of rapid testing kits across hospitals, STD clinics, and community healthcare settings, thereby driving regional market growth.
Asia Pacific is emerging as a high-growth region for the Trichomonas Rapid Testing Market, with China accounting for nearly 9.6% of the global market share in 2025. The COVID-19 pandemic had a significant yet temporary impact on sexually transmitted disease (STD) surveillance and control in the country. Strict lockdowns and healthcare system reprioritization disrupted routine screening, diagnosis, and prevention of STDs, including trichomoniasis. Several recent studies indicate that COVID-19 containment policies unintentionally reduced access to STD services, allowing infections to go underdiagnosed and untreated. This disruption increased the long-term public health burden and placed additional strain on medical services post-pandemic. As healthcare systems normalize, renewed focus on STD prevention, improved disease surveillance, and expanded access to rapid diagnostic testing are accelerating market growth. Increased government attention toward infectious disease control and rising awareness of asymptomatic STIs further support adoption of Trichomonas rapid testing across the region.
The Trichomonas rapid testing market is moderately consolidated, with established diagnostic companies holding a significant share due to strong brand presence, regulatory approvals, and wide distribution networks. Key players focus on technological advancements in rapid and point-of-care diagnostics to enhance test accuracy, speed, and usability. Strategic acquisitions and partnerships with local manufacturers enable expansion into developing markets and strengthen regional sales channels. Companies are also aligning product portfolios with evolving clinical practices and screening protocols to maintain competitiveness. Continuous innovation, geographic expansion, and portfolio diversification remain core strategies shaping the competitive structure of the market.?
The global Trichomonas Rapid Testing Market is expected to reach US$ 504.4 million in 2026.
Rising trichomoniasis prevalence, with 156 million annual cases per WHO, and POCT adoption fuel demand.
North America leads with 37% share in 2025, driven by US STI rates and regulations.
Molecular advancements in Asia Pacific offer expansion amid rising infections and manufacturing growth.
Leaders include Sekisui Diagnostics, Cepheid, Quidel Corporation, BD, and Hologic, Inc.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn Volume (Units) If Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
Product Type
Sample
End User
Regions
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author