ID: PMRREP22579| 199 Pages | 29 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

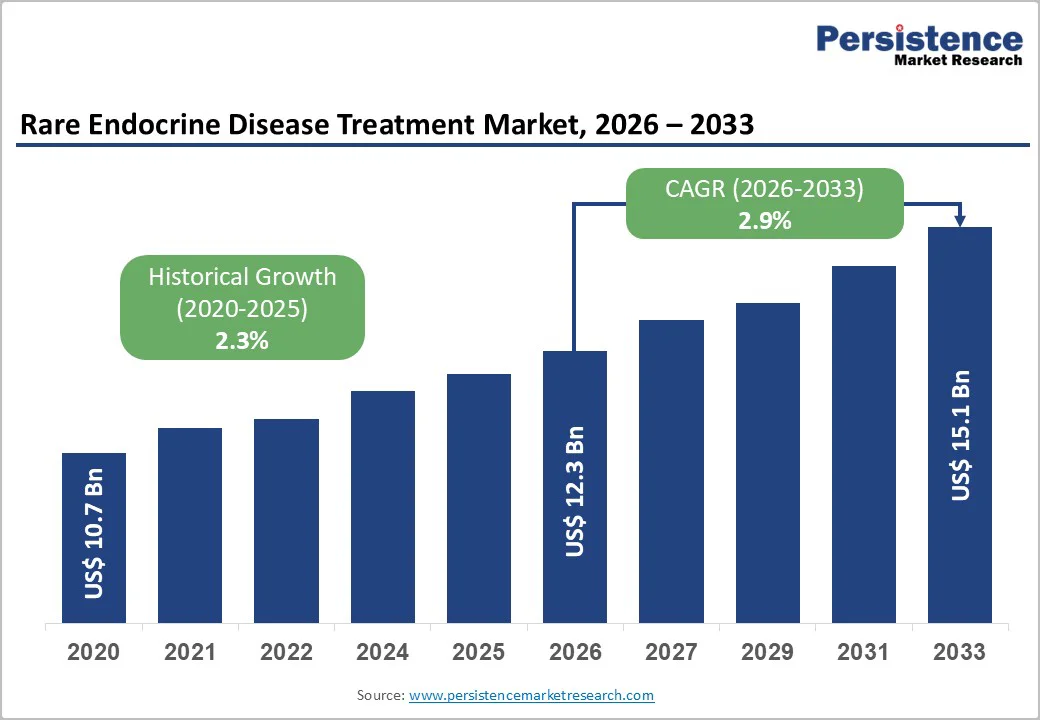
The global rare endocrine disease treatment market is projected to reach US$12.3 billion in 2026 and US$14.7 billion by 2033, growing at a CAGR of 2.9% from 2026 to 2033. The global market is growing rapidly, driven by the rising prevalence of conditions such as acromegaly, Cushing's syndrome, hypoparathyroidism, and Addison's disease. Increased awareness, improved diagnostics, and advances in hormone replacement, biologics, and targeted therapies are fueling demand.
Emerging orphan drugs, precision medicine, and increased funding for research support growth. The market is expanding as more patients gain access to specialized treatments, and healthcare infrastructure improves globally. Rising investment in innovative therapies and supportive government initiatives further propel market development, making rare endocrine disease treatment a high-priority segment in specialty pharmaceuticals.
| Key Insights | Details |
|---|---|
|
Rare Endocrine Disease Treatment Market Size (2026E) |
US$12.3 Bn |
|
Market Value Forecast (2033F) |
US$15.1 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
2.9% |
|
Historical Market Growth (CAGR 2020 to 2025) |
2.3% |
Genomic-Guided Therapy Adoption is rapidly transforming the treatment landscape for rare endocrine diseases by enabling highly personalized care. With advances in genetic sequencing and molecular diagnostics, clinicians can now identify precise mutations, hormonal imbalances, or receptor defects underlying complex endocrine disorders. This precise genetic insight enables treatment plans to move away from one-size-fits-all approaches toward individualized strategies that target the root cause of the disease rather than just managing symptoms. For patients with rare endocrine conditions, such as congenital adrenal hyperplasia, hypoparathyroidism, or rare pituitary disorders, genomic-guided therapies offer the possibility of better efficacy, reduced side effects, and improved long-term outcomes.
Additionally, the integration of genomics into clinical practice accelerates early diagnosis, often before irreversible damage occurs, and supports proactive monitoring. The growing accessibility of genetic testing, combined with expanding databases of rare mutations and their clinical implications, empowers endocrinologists to make data-driven decisions. As awareness of genomics’ role in rare endocrine disorders increases, adoption of such therapies is expected to rise sharply, shaping the future of precision medicine in this niche but critical market.
One significant challenge in the rare endocrine disease treatment market is the inherent complexity of therapy administration. Many of the preferred treatments, particularly biologics and peptide-based therapies, require specialized delivery methods such as intravenous infusions, subcutaneous injections, or hospital-administered protocols. These therapies cannot be self-administered easily at home, demanding access to trained medical professionals and well-equipped healthcare facilities. The complexity of administration often necessitates careful dosing schedules, monitoring for potential adverse effects, and managing immune or hormonal responses, all of which require close supervision.
This level of complexity can significantly impact patient compliance, as frequent hospital visits or prolonged infusion sessions can be burdensome, particularly for patients living in remote areas. It also places additional logistical and operational pressure on hospitals and treatment centers, which must maintain trained staff, specialized equipment, and cold-chain storage for sensitive biologic medications. Furthermore, this complexity may limit broader adoption of these therapies, particularly in regions with constrained healthcare infrastructure, thereby slowing market penetration despite their proven clinical efficacy.
Telemedicine and remote patient monitoring are transforming the management of rare endocrine disorders by bridging the gap between patients and specialized care, particularly in underserved or geographically remote regions. Patients with rare hormonal conditions often require frequent monitoring of hormone levels, regular dose adjustments, and timely medical consultations, which can be challenging to access in areas lacking endocrine specialists. Telehealth platforms enable continuous real-time monitoring of vital biochemical parameters, symptom tracking, and adherence to complex treatment regimens, allowing physicians to intervene promptly when abnormalities are detected. These platforms also facilitate virtual consultations, reducing the need for travel and associated stress for patients and caregivers.
Integration with mobile apps and wearable devices ensures seamless communication between healthcare providers and patients and provides personalized insights into therapy effectiveness. By improving accessibility, compliance, and early detection of complications, telemedicine not only enhances patient outcomes but also expands the overall market reach for rare endocrine disease treatments, creating new opportunities for digital health adoption.
Biologics account for the largest share of the rare endocrine disease treatment market because they offer targeted, highly effective therapies for conditions that conventional small-molecule drugs often cannot address. Many rare endocrine disorders result from genetic mutations or hormone deficiencies, which require precise intervention at the molecular or cellular level. Biologics, such as monoclonal antibodies, peptide-based therapies, and recombinant hormones, can directly replace missing hormones, modulate abnormal signaling pathways, or correct specific defects, leading to better clinical outcomes and reduced side effects. In contrast, organic compounds, including small-molecule drugs, generally act more broadly, managing symptoms rather than addressing root causes, which limits their effectiveness for rare conditions.
Additionally, regulatory incentives such as orphan drug designations and expedited approval pathways encourage the development and adoption of biologics. Patient and physician preference for these targeted therapies, combined with advanced biotechnological innovations and the growing emphasis on personalized medicine, further consolidates biologics as the dominant segment.
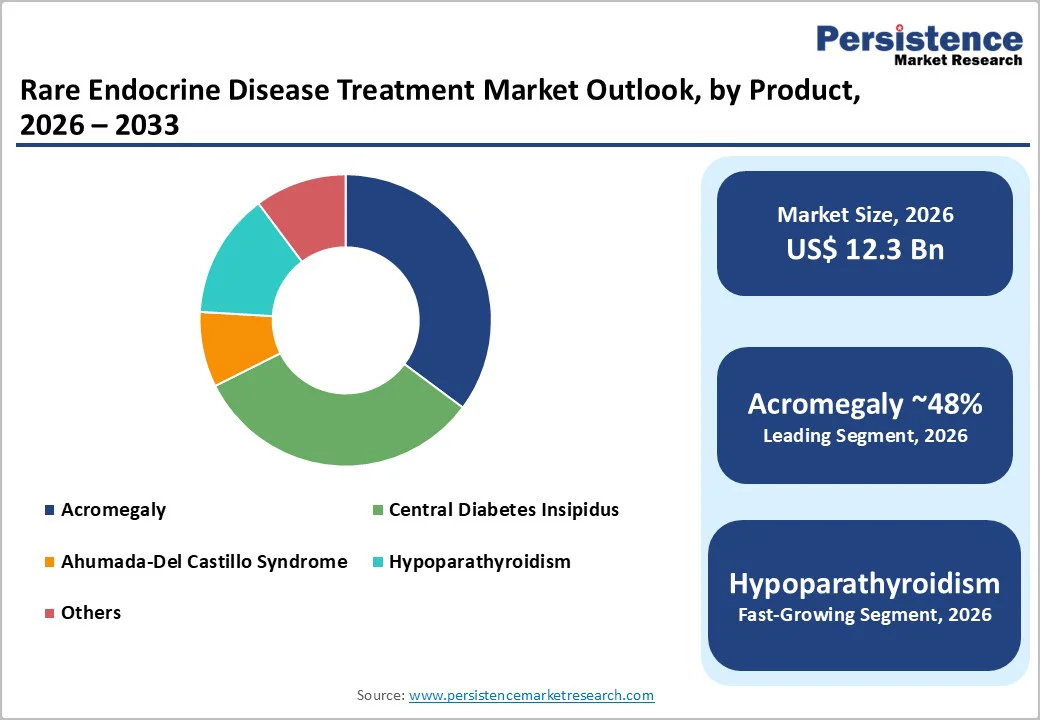
Acromegaly accounts for the highest market share because it has a comparatively higher diagnosed prevalence among rare endocrine conditions and requires lifelong medical management. The disease is driven by excess growth hormone, which leads to progressive complications involving bones, organs, metabolism, and cardiovascular functions, making treatment essential rather than optional. It has well-established diagnostic pathways through IGF-1 levels and imaging, allowing earlier identification than many ultra-rare endocrine disorders.
The standard of care often includes long-term biologic therapies such as somatostatin analogs, GH-receptor antagonists, and dopamine agonists, creating recurring treatment demand. In addition, specialist endocrinology clinics actively track and manage acromegaly cases due to associated comorbidities such as diabetes, sleep apnea, and hypertension, increasing treatment continuity. Compared with other listed conditions that may remain underdiagnosed or have short-term intervention needs, acromegaly requires structured, ongoing therapy, thereby contributing most to total treatment market revenue.
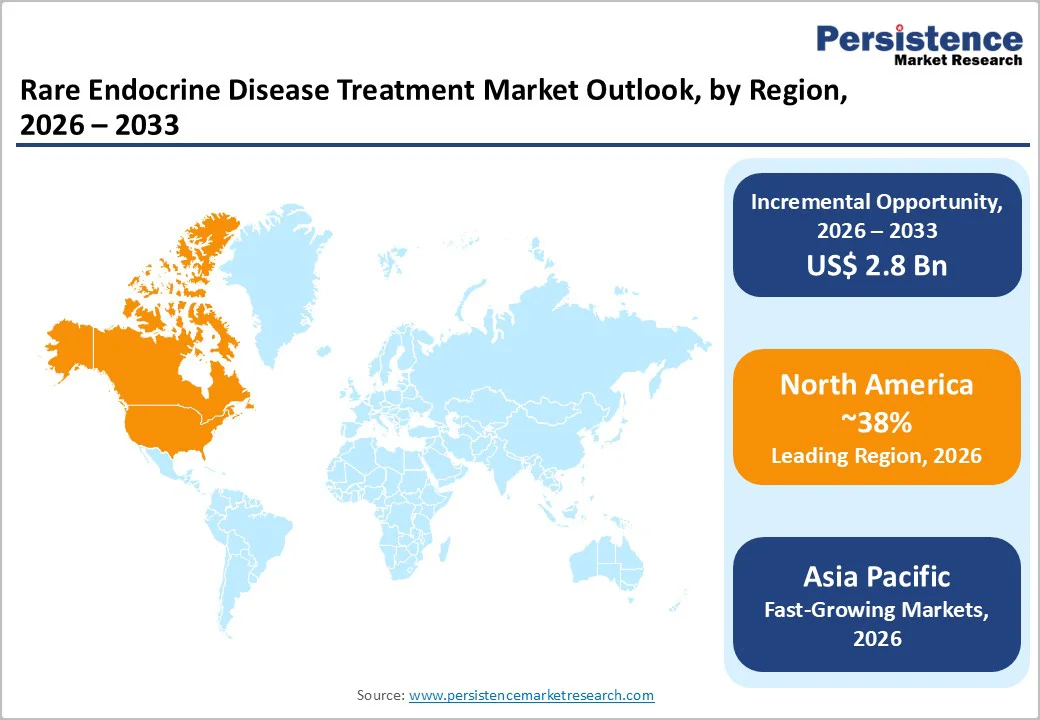
North America remains the leading hub for rare endocrine disease treatment, driven by early diagnosis capabilities, advanced clinical protocols, and highly specialized endocrine care networks. The region benefits from strong patient registries, genetic screening programs, and structured referral systems, enabling faster identification of hormone-related disorders that often go unnoticed elsewhere. In the United States, the presence of major biopharmaceutical innovators accelerates the development of biologics, recombinant hormone therapies, long-acting formulations, and novel immunomodulators, strengthening treatment availability.
Favorable reimbursement frameworks and orphan drug exclusivity create commercial viability for therapies that target small patient pools. U.S. hospitals and research institutions conduct active real-world evidence studies, shaping precision-based treatment models and digital monitoring tools for long-term endocrine management. Additionally, patient advocacy networks and online physician–patient platforms enhance awareness and adherence, contributing to high treatment continuity. These integrated strengths position the U.S. and the broader North American ecosystem as the most influential centers of growth and innovation in rare endocrine diseases.
Asia Pacific is emerging as a rapidly expanding market for rare endocrine disease treatments, driven by improved diagnostic access, greater specialist availability, and rising awareness among clinicians and patients. Several countries, including India, China, Japan, and South Korea, are investing heavily in endocrine research, genetic testing programs, and digital health platforms that support early detection. Local pharmaceutical manufacturers are increasingly developing biosimilars, long-acting hormone therapies, and cost-effective biologics, reducing treatment barriers and improving affordability.
Government-supported policies and funding initiatives are helping expand access to specialty therapies that were previously limited to Western regions. Medical tourism hubs and tertiary hospitals in Asia are also attracting regional patients seeking specialized endocrine interventions. In parallel, the growing burden of metabolic disorders and improved screening are expanding the rare endocrine patient population. Together, these advancements position the Asia-Pacific as a strong emerging market with accelerating therapeutic adoption and infrastructure growth.
The competitive landscape of the rare endocrine disease treatment market is characterized by innovation-focused players expanding biologics, hormone analogs, and long-acting formulations. The market features a strong pipeline of targeted therapies addressing genetic and hormone-driven abnormalities, with emphasis on improved efficacy and limited side effects. Strategic collaborations, licensing agreements, and investments in orphan drug development influence market positioning. Digital monitoring tools, patient support programs, and precision-diagnostic partnerships further differentiate offerings.
The global rare endocrine disease treatment market is projected to be valued at US$12.3 Bn in 2026.
The rare endocrine disease treatment market is driven by increasing diagnosis of hormone-related disorders through advanced biochemical and genetic testing, along with rising patient awareness supported by specialty endocrinology centers.
The global rare endocrine disease treatment market is poised to witness a CAGR of 2.9% between 2026 and 2033.
Expansion of telemedicine and remote patient monitoring enables access to specialist care in underserved regions. Growth in biologics, peptide-based therapies, and long-acting formulations provides differentiation and improved outcomes.
Novartis, Ipsen, Pfizer, Inc., Teva Pharmaceutical Industries Limited, EMD serono, and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Drug Type
By Indication
By Mode of Administration
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author