ID: PMRREP34757| 198 Pages | 28 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

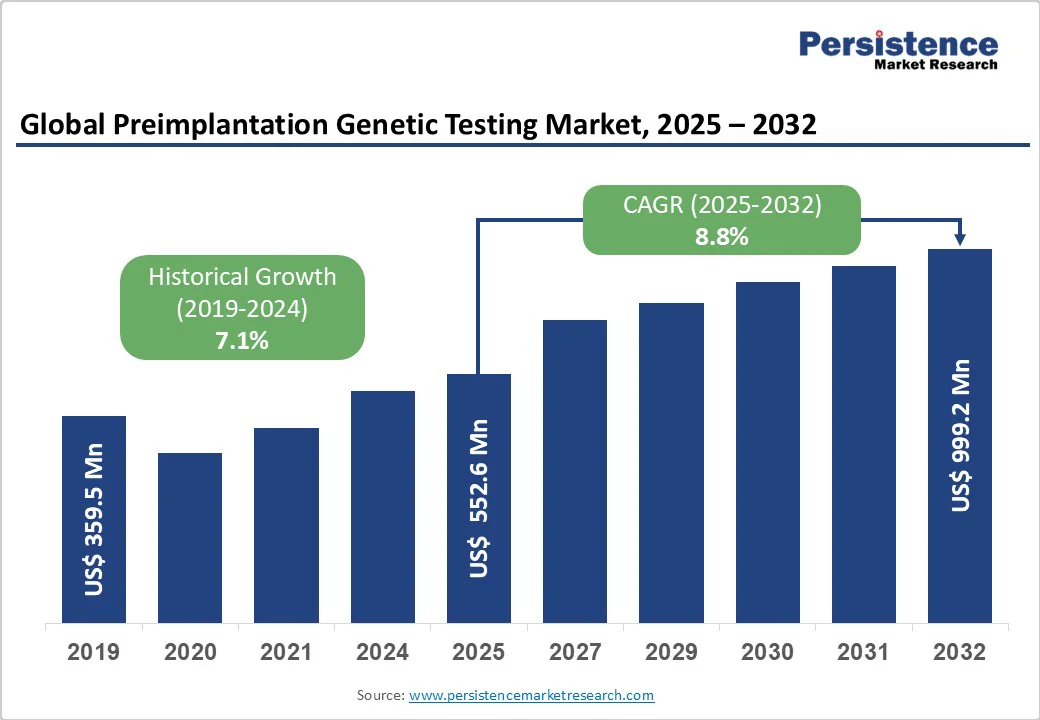
The global preimplantation genetic testing market is anticipated to reach a value of US$552.7 million in 2025 and reach a value of US$999.2 million at a CAGR of 8.8% by 2032.
The preimplantation genetic testing (PGT) market is experiencing steady growth, driven by the rising prevalence of genetic disorders and increased adoption of in vitro fertilization (IVF) procedures. Technological advancements, such as next-generation sequencing and improved embryo biopsy techniques, have enhanced test accuracy and reliability. Growing awareness among the parents about hereditary diseases and increasing maternal age are also fueling demand. Supportive regulatory frameworks and improved healthcare infrastructure, especially in developed regions, further contribute to market expansion. Additionally, the availability of user-friendly testing kits and automated platforms is streamlining clinical workflows, making PGT more accessible and efficient in fertility treatments.
| Key Insights | Details |
|---|---|
|
Preimplantation Genetic Testing Market Size (2025E) |
US$ 552.7 Mn |
|
Market Value Forecast (2032F) |
US$ 999.2 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
8.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.3% |
The trend of increasing maternal age is a major contributor to rising infertility rates globally. Many women now delay childbearing due to career, financial, or personal reasons. In England and Wales, the average age of first-time mothers rose from 23 in 1970 to 29 in 2020. Fertility declines with age: women in their early 30s have about a 20% chance of conceiving per cycle, dropping to 15% by age 35 and below 5% in the early 40s.
This decline has led to greater reliance on assisted reproductive technologies (ART), especially in vitro fertilization (IVF). In the UK, IVF accounts for over 99% of ART procedures. In 2021, 86,146 babies about 2.3% of all births were conceived through ART. IVF success rates also decrease with age: 31% for women under 35, 16% for those 38–40, and just 3% for women over 44.
To improve IVF outcomes and reduce genetic risks, preimplantation genetic testing (PGT) is increasingly used. PGT screens embryos for chromosomal abnormalities before implantation. In the U.S., around 16.6% of ART cycles involve PGT. Demand is growing due to rising infertility and awareness of genetic disorders. In India, preimplantation genetic diagnosis (PGD), a form of PGT, is regulated by the Pre-Conception and Pre-Natal Diagnostic Techniques (PCPNDT) Act of 1994. This law prohibits sex selection and aims to prevent misuse of prenatal diagnostics.
In summary, delayed childbearing is driving higher infertility rates and demand for ART and PGT, offering hope to couples while reducing genetic risks linked to advanced maternal age.
Preimplantation Genetic Testing (PGT), while valuable for detecting chromosomal abnormalities, faces several restraints limiting its broader adoption. One key concern is the potential for embryo damage during the biopsy process, where cells are removed from the trophectoderm layer at the blastocyst stage. Though generally considered safe, studies in Human Reproduction and Fertility and Sterility have linked this procedure to increased pregnancy complications, including a nearly threefold rise in preeclampsia risk and a slightly elevated risk of preterm birth. Diagnostic inaccuracy also undermines trust in PGT. False positives can result in discarding viable embryos.
A 2019 study found that 49.3% of embryos classified as abnormal by PGT-A led to healthy live births upon transfer. Mosaicism further complicates results, with only 42% of retested embryos yielding consistent classifications. Ethical and legal concerns add another layer of restraint. In Australia, Monash IVF settled a $56 million class-action lawsuit involving over 700 patients due to alleged misclassifications from non-invasive PGT-A, highlighting the real-world consequences of diagnostic errors. These risks underscore the need for stringent clinical oversight, enhanced testing accuracy, and thorough patient counseling, which currently hinder widespread PGT market growth.
Technological innovations in genetic analysis are creating major opportunities for enhancing Preimplantation Genetic Testing (PGT) and improving in vitro fertilization (IVF) outcomes. Next-generation sequencing (NGS) enables high-resolution chromosomal analysis, significantly boosting pregnancy and live birth rates, particularly in women of advanced maternal age. In a study of 1,099 couples, NGS-based PGT-A increased the clinical pregnancy rate from 45.6% to 54.4%, and the live birth rate from 30.9% to 52.1%, while reducing miscarriage rates. Artificial intelligence (AI) further transforms PGT by analyzing embryo images to predict viability. Models such as convolutional neural networks (CNNs) and PIMS-AI have achieved high predictive accuracy, with some showing an AUC of 0.90.
Additionally, non-invasive PGT methods using cell-free DNA from spent culture media minimize embryo damage while maintaining diagnostic accuracy. These techniques, especially when integrated with AI, enhance embryo selection, reduce costs, and increase IVF success rates, presenting strong growth opportunities for the PGT market.
Kits hold a prominent position in the preimplantation genetic testing (PGT) market, primarily due to their ability to simplify and standardize the testing workflow. These kits are user-friendly and come with pre-validated reagents and optimized protocols, ensuring consistent and accurate genetic analysis during IVF procedures. By minimizing the risk of human error and reducing reliance on specialized expertise, kits enable broader adoption in fertility clinics. As demand for IVF services grows, clinics are increasingly turning into efficient, scalable solutions, making kits an ideal choice. Their compatibility with automated systems and availability in both advanced and emerging healthcare markets further enhance their adoption, reinforcing their importance within the PGT landscape.
Next-Generation Sequencing (NGS) has emerged as the leading technology in the Preimplantation Genetic Testing (PGT) market due to its high accuracy, efficiency, and comprehensive analysis capabilities. NGS allows simultaneous screening of multiple embryos for chromosomal abnormalities, genetic mutations, and structural variations, providing more reliable results compared to traditional methods. Its ability to detect even subtle genetic changes enhances embryo selection, improving success rates in assisted reproductive technologies. Additionally, declining costs of sequencing, faster turnaround times, and growing adoption by fertility clinics worldwide are further driving market preference for NGS. The technology is particularly valued for personalized and precise reproductive planning.
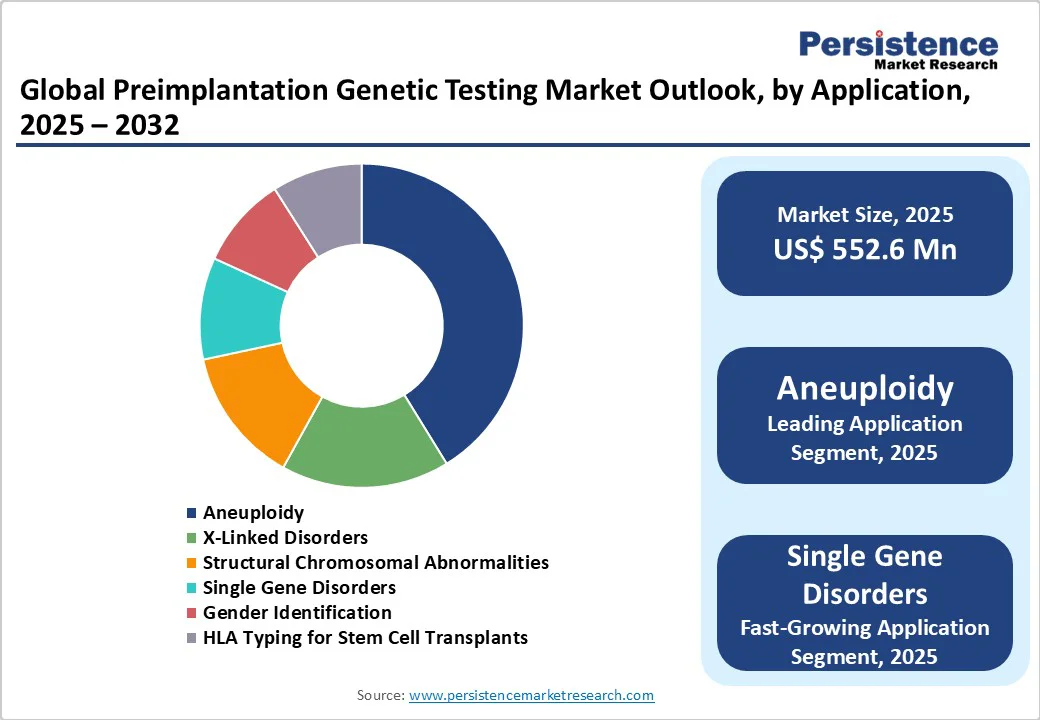
In 2024, the aneuploidy application segment led the preimplantation genetic testing (PGT) market, capturing a substantial 41.0% revenue share. This dominance is attributed to the high prevalence of chromosomal abnormalities, particularly among women of advanced maternal age, and the growing demand to prevent implantation failure and miscarriage.
Aneuploidy testing improves embryo selection by identifying abnormal chromosome numbers, enhancing IVF success rates. It is also the fastest-growing segment, driven by advancements in next-generation sequencing and increasing awareness of genetic screening benefits. Rising acceptance of PGT-A in clinical practice further supports its rapid adoption and sustained market expansion.
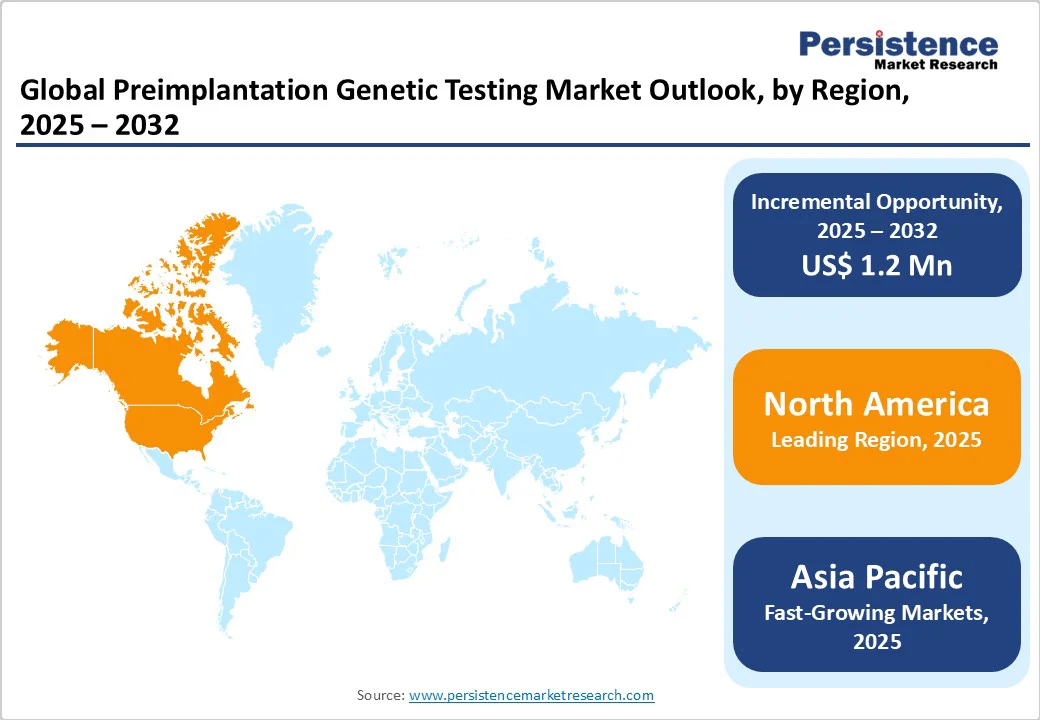
North America holds the largest share in the preimplantation genetic testing (PGT) market, with the U.S. preimplantation genetic testing market emerging as the leading contributor within the region. This growth is fueled by the widespread adoption of advanced reproductive technologies, increasing awareness of genetic screening, and the presence of leading fertility clinics and PGT providers. The region benefits from significant investment in genetic research and favorable reimbursement policies that enhance access to fertility treatments. A growing number of technologically advanced fertility clinics further supports market expansion. Rising maternal age and a higher prevalence of genetic disorders are also increasing the demand for early genetic screening.
Moreover, strong regulatory support and active collaborations between academic institutions and diagnostic companies foster continuous innovation. These factors collectively position North America as a global leader in both the technological development and clinical application of PGT.
The Europe preimplantation genetic testing market is experiencing steady growth, primarily driven by the increasing adoption of in vitro fertilization (IVF) procedures and heightened awareness of hereditary genetic disorders among prospective parents. Advances in genetic screening technologies, including next-generation sequencing and comprehensive chromosomal analysis, are improving test accuracy, reducing turnaround times, and enhancing overall clinical efficiency. Favorable regulatory frameworks across key European countries support safe and standardized implementation of PGT, encouraging wider adoption. The UK is expected to be the fastest growing country between 2025 and 2032, fueled by growing demand for fertility treatments and supportive healthcare policies. Additionally, the presence of leading diagnostic companies and specialized service providers promotes continuous innovation, expands service accessibility, and strengthens market competitiveness, further driving the growth and development of the European PGT market.
Asia Pacific offers substantial growth potential for the preimplantation genetic testing (PGT) market, fueled by increasing access to fertility care and a growing incidence of genetic disorders. Rising awareness of assisted reproductive technologies and changing social norms around delayed parenthood are driving demand for advanced fertility treatments. Countries such as China, India, and Japan are witnessing rapid urbanization and significant improvements in healthcare infrastructure, creating a strong foundation for market development.
Supportive government policies for infertility treatments and increased investment in genomics research are further accelerating progress. Moreover, the rise of local manufacturers providing cost-effective kits and reagents is improving affordability and access, enabling wider adoption of PGT across various economic and demographic groups.
The preimplantation genetic testing (PGT) market features a dynamic competitive landscape, comprising both established diagnostic companies and emerging biotech firms. Key players are heavily focused on technological advancements, particularly in next-generation sequencing (NGS) and AI-driven embryo analysis, to improve the precision and efficiency of testing. Strategic mergers, acquisitions, and collaborations are prevalent as companies seek to broaden their product offerings and expand their global footprint. There is also increasing competition in the development of user-friendly kits and automated platforms designed to meet the operational needs of fertility clinics.
The global market is estimated to increase from US$ 552.7 million in 2025 to US$ 999.2 million in 2032.
Rising IVF procedures, increased genetic disorder awareness, technological advancements, and growing maternal age are key drivers of the PGT market growth.
The market is projected to record a CAGR of 8.8% during the forecast period from 2025 to 2032.
Thermo Fisher Scientific, Inc., COOPER SURGICAL, INC., Illumina, Inc., Abbott, Agilent Technologies, Inc., and Others.
Opportunities lie in AI integration, non-invasive testing, expanding fertility clinics in emerging markets, and improved accessibility through cost-effective testing solutions.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn, Volume: As applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Technology
By Application
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author