ID: PMRREP12784| 196 Pages | 4 Dec 2025 | Format: PDF, Excel, PPT* | Chemicals and Materials

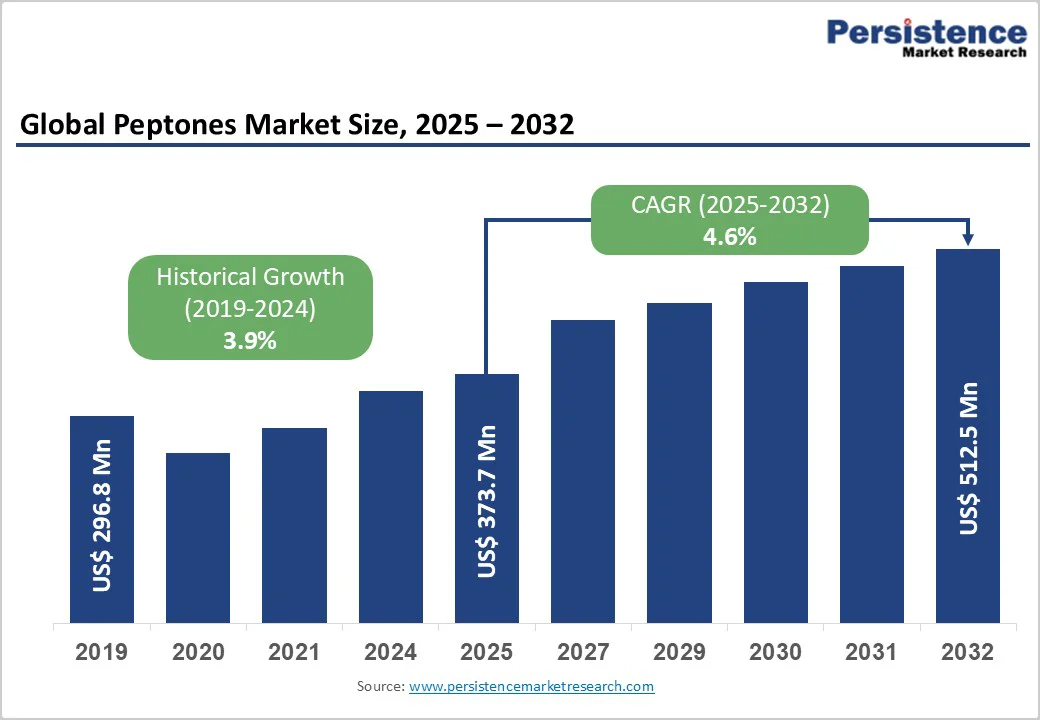
The global peptones market size is valued at US$ 373.7 million in 2025 and is projected to reach US$ 512.5 million by 2032, growing at a CAGR of 4.6% between 2025 and 2032. The global peptone industry is experiencing a fundamental transformation driven by sustainability, regulatory compliance, and innovation.
Manufacturers are steadily transitioning away from animal-derived sources such as casein and meat extracts toward plant-, microbial-, and recombinant-based alternatives that offer safer, more consistent, and ethically aligned production.
Heightened regulatory scrutiny-especially in pharmaceuticals and diagnostics-has intensified the demand for BSE (Bovine Spongiform Encephalopathy)-free, TSE (Transmissible Spongiform Encephalopathy)-free, and GMO (Genetically Modified Organism)-free certifications, compelling suppliers to implement stringent traceability and quality systems.
| Key Insights | Details |
|---|---|
| Global Peptones Market Size (2025E) | US$ 373.7 Million |
| Market Value Forecast (2032F) | US$ 512.5 Million |
| Projected Growth (CAGR 2025 to 2032) | 4.6% |
| Historical Market Growth (CAGR 2019 to 2024) | 3.9% |
The global peptone market is witnessing strong growth, primarily driven by the rising demand for microbial cultures across medical research, pharmaceuticals, agriculture, and food safety.
The surge in infectious diseases such as tuberculosis, HIV, and COVID-19 has amplified the need for reliable microbiological testing, accelerating peptone adoption as a key nutrient source for microbial growth. Derived from protein hydrolysis, peptone supplies essential nitrogenous, carbonaceous, and amino compounds that support bacteria and fungi cultivation and carbohydrate fermentation studies.
In medical research, peptones enable the study of pathogens; in food safety, they help detect contamination; and in biopharmaceuticals, they boost cell growth for vaccine and therapeutic production.
According to Thermo Fisher’s white paper “Peptones: Established Supplements for Vaccine Applications,” peptones enhance cell viability and viral titers, reducing time-to-market for vaccine pipelines. Their use in over 150 marketed biologics, including 15 blockbuster drugs, underscores regulatory familiarity and manufacturing scalability.
As serum alternatives gain traction due to cost and supply challenges, high-performance peptones -offering low endotoxin levels and GMP-grade consistency- are emerging as strategic bioprocessing inputs, ensuring reproducibility, efficiency, and compliance.
Despite their low base production cost, peptones face substantial restraints in high-value pharmaceutical, biotech, and food applications due to regulatory and safety challenges.
Scaling peptones for GMP-grade or endotoxin-free use demands advanced purification methods such as ultrafiltration and depyrogenation, alongside extensive validation processes to meet Food and Drug Administration (FDA) and European Medicines Agency (EMA) standards-greatly inflating production costs.
Animal-derived variants pose additional hurdles, including testing for microbial contaminants, endotoxins, and transmissible pathogens including BSE/TSE, increasing both timelines and batch rejection risks.
In pharmaceuticals, the growing shift toward recombinant growth factors and chemically defined media further limits peptone adoption, as these alternatives provide superior consistency and regulatory acceptance. In the food and beverage industry, allergen-related concerns amplify constraints.
Many peptones are derived from soy, wheat, dairy, or egg proteins, triggering strict scrutiny under tightening allergen labeling and safety regulations enforced by the FDA, European Food Safety Authority (EFSA), Food Safety and Standards Authority of India (FSSAI), and Ministry of Food and Drug Safety (MFDS-South Korea).
Cross-contamination risks during manufacturing add further complexity. Consequently, the need for allergen-free certification, combined with high compliance costs and regulatory risks, significantly limits peptone use in sensitive applications such as infant nutrition, medical foods, and advanced biologics.
The rapidly advancing cultivated meat industry presents a major growth opportunity for the global peptone market, driven by the need for cost-effective, ethical, and consistent growth media.
Traditional animal-derived peptones-such as casein peptone from milk proteins and collagen peptone from connective tissues-contain 80-90% amino acids and have long served as key nitrogen sources in microbial and cell culture. However, their animal origin poses biosafety risks such as Bovine Spongiform Encephalopathy (BSE) and swine fever, while also excluding vegan, halal, kosher, and culturally sensitive markets.
This has accelerated demand for animal-free, food-grade peptones derived from plants, yeast, algae, and recombinant microbial sources. These alternatives eliminate zoonotic risks, improve sustainability, and align with rising consumer preferences for cruelty-free food systems.
Moreover, they enable serum-free and chemically defined media formulations-reducing variability and enhancing regulatory acceptance.
Companies are increasingly investing in fermentation-based recombinant technologies to engineer next-generation peptones with customized amino acid profiles optimized for muscle or adipocyte cell growth. As cultivated meat scales toward commercialization, such innovations are poised to position animal-free peptones as critical enablers of sustainable, safe, and globally acceptable protein production.
Animal-derived peptones are expected to account for 54.1% of the global market by 2025, driven by their superior amino acid and peptide composition that supports robust microbial growth.
Their proven efficacy across pharmaceutical, diagnostic, and food microbiology applications continues to make them the preferred choice despite growing ethical and regulatory scrutiny. Strong performance consistency and wide availability from casein, meat, and gelatin sources further reinforce their dominance in large-scale bioprocessing and culture media production.
Protein hydrolysates are projected to capture 68.1% of the global peptone market by 2025, owing to their high solubility, balanced nutrient profile, and suitability across diverse fermentation and cell culture processes. Their ability to provide peptides, amino acids, and growth factors in bioavailable forms enhances cell metabolism and yield.
Widely used in pharmaceuticals, diagnostics, and food biotechnology, protein hydrolysates ensure consistent performance, making them the most reliable and cost-efficient option for large-scale microbial and mammalian cell culture applications.
Microbial cell culture is projected to account for 77.1% of the global market by 2025, driven by its indispensable role in vaccine production, antibiotic testing, and enzyme manufacturing. Peptones serve as the key nutrient source enabling microbial growth and metabolic activity across biopharma and clinical microbiology laboratories.
Rising demand for biologics, biosimilars, and rapid diagnostics has further intensified reliance on high-quality peptones to ensure consistent growth conditions, reproducibility, and regulatory compliance across industrial and research-grade fermentation systems.
Biopharmaceutical companies are expected to hold 34.1% of the global peptone market by 2025, fueled by growing demand for monoclonal antibodies, vaccines, and recombinant therapeutics. Peptones play a crucial role in cell culture media formulation and microbial fermentation, enhancing cell viability and product yield.
With the biopharma sector increasingly shifting toward serum-free and defined media systems, consistent and high-performance peptones have become essential for ensuring regulatory-compliant, scalable, and efficient biologics manufacturing processes.
Powder-based peptones are projected to represent 78.7% of the global market by 2025, owing to their longer shelf life, transport convenience, and better compatibility with automated processing systems. Their stable composition allows precise dosing and uniform distribution in media formulations.
Powder forms are widely preferred in pharmaceutical, diagnostic, and academic research laboratories due to ease of reconstitution, low contamination risk, and scalability advantages in both small- and large-scale fermentation setups.
Non-ultra-filtered (standard) peptones are projected to command 80.0% of the global market by 2025, supported by their affordability and suitability for routine microbial culture and fermentation processes. While ultra-filtered grades cater to specialized biopharma applications, standard peptones meet the nutritional and quality requirements of most industrial and academic research users.
Their balanced nutrient composition, ease of availability, and compatibility across a wide range of microbial species sustain their dominance in global laboratory and industrial microbiology workflows.
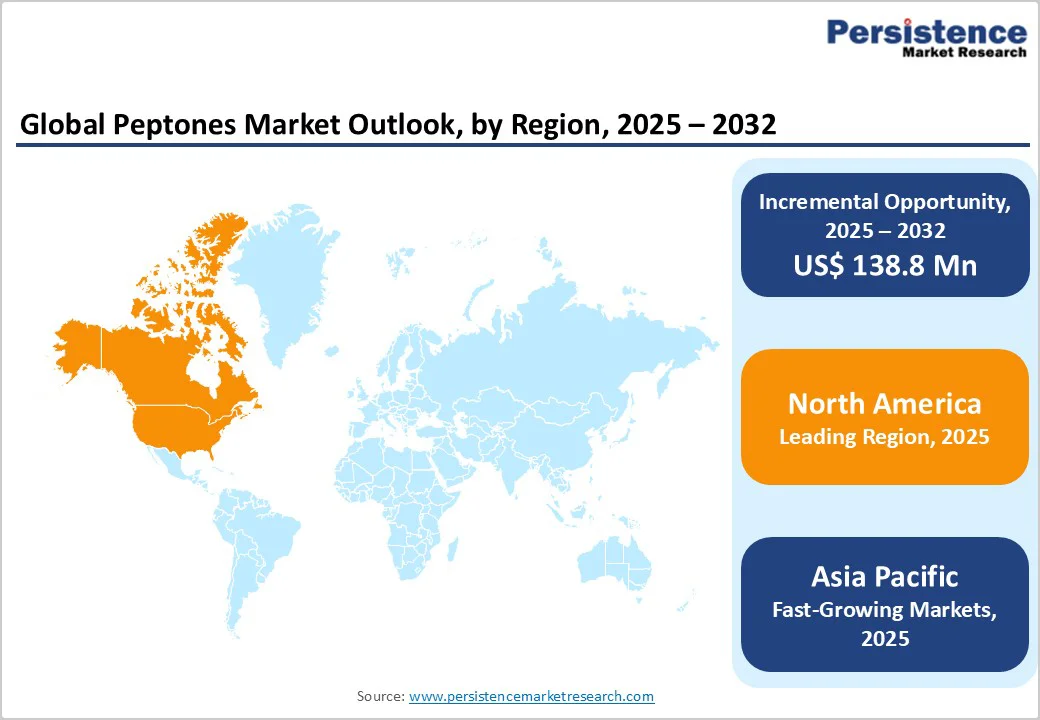
By 2025, North America is projected to account for nearly 33.8% of the global peptone market, supported by strong growth in diagnostics, biopharmaceutical production, and clean-label food applications.
Clinical microbiology laboratories, hospitals, and research institutes remain major demand drivers, relying on high-purity medical-grade peptones for cultivating bacteria, fungi, and other microorganisms essential for disease detection, antibiotic sensitivity testing, and vaccine development. The expansion of rapid diagnostic and point-of-care testing further amplifies demand for standardized peptone formulations that ensure accuracy and reproducibility.
Simultaneously, the region’s growing vegan and clean-label movement is reshaping food and nutraceutical formulations. Manufacturers are increasingly adopting plant-derived peptones from soy, pea, wheat, and corn as sustainable alternatives to animal-based sources such as casein or meat extracts. These vegan-certified variants align with consumer preferences for allergen-free, ethical, and environmentally responsible products.
In October 2024, Merck reinforced regional biomanufacturing strength by opening a €290 million biosafety testing facility in Rockville, Maryland, enhancing global drug development and testing capabilities. Collectively, the region’s advanced healthcare infrastructure, innovation-driven food sector, and strong regulatory compliance continue to position North America as a key growth engine in the global peptone market.
By 2025, Europe is expected to capture nearly 25.8% of the global peptone market, supported by expanding biotechnology research, strategic partnerships, and strong industrial integration across the region. The market’s growth is fueled by rising demand for high-quality culture media components in biopharmaceutical, diagnostic, and food applications.
Key industry collaborations are reinforcing supply networks and innovation capacity. In June 2024, Nu-Tek BioSciences inked a deal with IMCD for distribution, expanding its global reach in Europe and the Asia-Pacific regions. It executed an agreement for distributing peptones and hydrolysates, strengthening its position as a market leader in animal-free ingredients.
IMCD, a global distributor and formulator of specialty chemicals and ingredients, enabled Nu-Tek to serve local customers while expanding IMCD’s product line. Similarly, in October 2024, Lesaffre acquired a majority stake in Biorigin, enhancing yeast derivative production and logistics to serve global food and biotech customers.
Further strengthening regional biomanufacturing, Solabia’s September 2024 acquisition of PolymerExpert bolstered its biomaterials portfolio, particularly in pharmaceuticals and cosmetics. Additionally, Kerry’s February 2023 partnerships with Azelis and Caldic streamlined its European distribution network, improving supply chain efficiency and service delivery.
Together, these strategic moves underscore Europe’s strong focus on innovation, quality control, and secure raw material sourcing-factors that continue to propel the region’s growing influence in the global peptone market.
The Asia Pacific market is expanding rapidly, projected to grow at a CAGR of 5.2% over the forecast period. The Asia-Pacific region-led by China, India, and South Korea-is rapidly emerging as a global biotechnology hub, driving robust demand for high-quality cell culture media and raw materials such as pharmaceutical-grade peptones.
Backed by strong government funding, a surge in academic and private R&D, and the rapid growth of biotech startups, the region’s biopharma landscape is expanding across biologics manufacturing, vaccine development, and biosimilar production.
Peptones, essential for microbial fermentation and cell culture, are increasingly consumed across these segments. However, despite rising demand, regional supply chains remain underdeveloped, with most manufacturers still relying on imports from Western suppliers-resulting in longer lead times, higher costs, and inconsistent availability.
This dependency creates a strategic opportunity for both local and global producers to establish regional manufacturing hubs, offering customized, compliant, and cost-effective peptone solutions.
Innovation is also emerging within other Asia countries such as South Korea, where researchers are developing marine-derived peptones from fish by-products like cutlassfish heads, while casein- and soy-based variants see wide adoption across laboratories.
Furthermore, the region’s expanding academic and contract research base, coupled with a shift toward precision medicine and biosimilars, is intensifying the need for consistent, high-performance peptones to support next-generation biologics and diagnostic research.
The global peptone market is witnessing robust expansion, supported by ongoing innovation, capacity enhancement, and certification-driven quality improvements. Companies are actively investing in advanced manufacturing facilities, sustainable ingredient development, and strategic distribution partnerships to strengthen global reach and reliability.
Overall, the competitive landscape reflects increasing consolidation, technological sophistication, and a strong push toward animal-free, high-performance peptone solutions tailored to biopharma, diagnostics, and food sectors.
The global market is projected to be valued at US$ 373.7 Million in 2025.
Rising demand for biologics, vaccines, and probiotics is driving consistent growth in the global peptones market.
The global market is poised to witness a CAGR of 4.6% between 2025 and 2032.
Expanding biopharmaceutical manufacturing and increasing adoption of animal-free peptones offer strong market opportunities.
Major players in the global are Merck KGaA, Kerry Inc., Thermo Fisher Scientific Inc., Tatua Co-operative Dairy Company Limited, Biotechnica and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Source
By Product
By Application
By Industry
By Form
By Grade
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author