ID: PMRREP31545| 242 Pages | 19 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

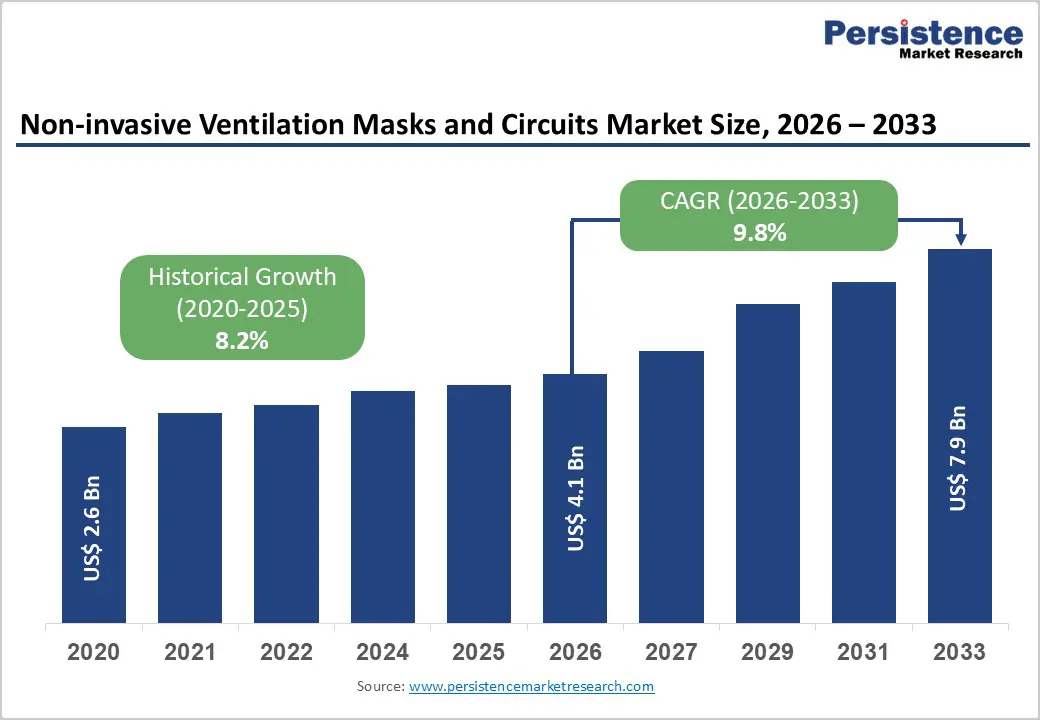
The global Non-invasive Ventilation Masks and Circuits market size is expected to be valued at US$ 4.1 billion in 2026 and projected to reach US$ 7.9 billion by 2033, growing at a CAGR of 9.8% between 2026 and 2033.
The primary drivers include the escalating burden of chronic respiratory diseases and technological advancements in patient-centric devices. WHO statistics reveal that respiratory diseases account for 4.1 million deaths annually, with COPD affecting over 384 million people worldwide, necessitating efficient non-invasive therapies. Furthermore, the aging global population, expected to double to 2.1 billion individuals over 60 by 2050 according to United Nations projections, amplifies demand for homecare solutions. Innovations such as integrated humidification and leak compensation features enhance efficacy, reducing hospital stays and costs while improving compliance.
| Global Market Attributes | Key Insights |
|---|---|
| Non-invasive Ventilation Masks and Circuits Size (2026E) | US$ 4.1 billion |
| Market Value Forecast (2033F) | US$ 7.9 billion |
| Projected Growth CAGR(2026-2033) | 9.8% |
| Historical Market Growth (2020-2025) | 8.2% |
Increasing Burden of Chronic Respiratory Disorders
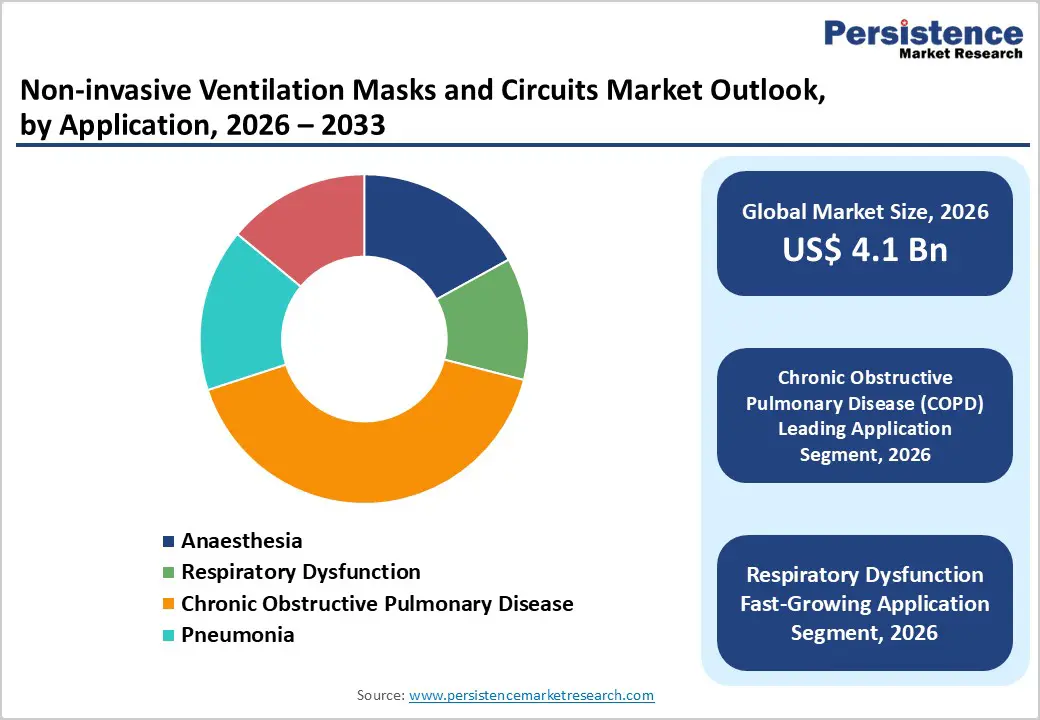
The rising incidence of chronic respiratory diseases such as Chronic Obstructive Pulmonary Disease (COPD) and acute respiratory distress syndrome represents a primary growth driver for the non-invasive ventilation masks and circuits market. According to the WHO, COPD is the third leading cause of death globally, with prevalence projected to increase by 23.3% from 480 million cases in 2020 to 592 million by 2050, driven by tobacco use, air pollution, and urbanization. Non-invasive ventilation (NIV) masks and circuits provide effective positive airway pressure therapy, reducing the need for invasive intubation, which carries 10-20% mortality risks in ICU settings. Clinical trials demonstrate NIV efficacy in managing COPD exacerbations, lowering rehospitalization rates by up to 50% and shortening hospital stays. This shift towards less invasive, cost-effective alternatives in both hospital and homecare settings directly correlates with sustained market expansion, as healthcare systems prioritize patient safety and resource optimization.
Technological Advancements and Patient Comfort Innovations
Advancements in material science and device design, including silicone-based masks with adaptive seals and lightweight circuits, significantly propel market growth by enhancing user comfort and therapy adherence. Post-COVID-19, demand surged for interfaces minimizing facial pressure sores, with ergonomic designs reducing discontinuation rates from 30% to under 10% in long-term users. Integration of heated humidifiers prevents rainout and mucociliary clearance issues, as evidenced by studies showing improved oxygenation. Portable battery-operated systems facilitate homecare, aligning with global trends towards ambulatory care. These innovations not only expand applications beyond acute care to chronic management but also support regulatory approvals for extended use, fostering broader adoption across demographics and reinforcing positive market momentum.
Market Restrains
Elevated Device Costs and Maintenance Expenses
Elevated device costs and ongoing maintenance expenses act as a significant restraint on the growth of the Non-invasive Ventilation (NIV) masks and circuits market. High-quality NIV masks and circuits are manufactured using advanced materials and precision designs to ensure patient comfort, durability, and leak prevention, which increases their upfront cost. In addition, frequent replacement of masks, headgear, tubing, and filters is required to maintain hygiene, prevent infections, and ensure optimal performance, further adding to the total cost of ownership. For hospitals and healthcare facilities, especially in low- and middle-income regions, budget constraints limit large-scale adoption and regular upgrades of NIV accessories. In homecare settings, high out-of-pocket expenses discourage long-term patient compliance, particularly for chronic conditions such as COPD and sleep-related breathing disorders that require continuous use. Maintenance costs related to cleaning, sterilization, and compatibility with different ventilator systems also increase operational complexity. These financial barriers collectively restrict wider adoption and slow market penetration, particularly in cost-sensitive healthcare systems.
Stringent Regulatory and Quality Compliance Requirements
Stringent regulatory and quality compliance requirements act as a significant restraint on the growth of the Non-invasive Ventilation (NIV) Masks and Circuits market. These products are classified as medical devices and must comply with rigorous regulatory frameworks such as U.S. FDA 510(k) clearance, CE marking under the EU Medical Device Regulation (MDR), and ISO standards related to biocompatibility, sterilization, and performance safety. Meeting these requirements involves extensive clinical validation, product testing, and detailed documentation, which significantly increases development timelines and costs. Smaller manufacturers and new entrants often face challenges in navigating complex regulatory pathways, limiting innovation and market entry. Additionally, frequent regulatory updates and post-market surveillance obligations require continuous quality monitoring and periodic product modifications, further raising operational expenses. Compliance with strict quality standards for materials, leak prevention, patient comfort, and infection control is especially critical for NIV masks and circuits used in intensive care and homecare settings. These regulatory burdens can delay product launches, increase pricing pressures, and restrict the availability of cost-effective solutions, thereby slowing overall market expansion despite rising demand for non-invasive respiratory support devices.
Market Opportunities
Emerging Opportunities in Homecare and Telemedicine Integration
Emerging opportunities in homecare and telemedicine integration are significantly expanding the growth potential of the Non-invasive Ventilation (NIV) masks and circuits market. The rising prevalence of chronic respiratory conditions such as COPD, sleep apnea, and neuromuscular disorders is driving a shift from hospital-based ventilation toward long-term homecare settings. Patients and healthcare systems increasingly prefer home-based NIV due to lower treatment costs, reduced hospital admissions, and improved patient comfort and quality of life. This trend is accelerating demand for user-friendly, lightweight, and comfortable NIV masks and durable, easy-to-maintain circuits designed specifically for home use. In parallel, the integration of telemedicine and remote patient monitoring technologies is creating new opportunities for market players. Smart NIV systems equipped with connected masks, sensors, and circuits enable real-time monitoring of patient adherence, breathing patterns, and therapy effectiveness. Clinicians can remotely adjust ventilation settings, provide timely interventions, and reduce the need for frequent hospital visits. As digital health infrastructure improves and reimbursement for home-based respiratory care expands, the adoption of connected NIV solutions is expected to grow rapidly, creating a strong long-term opportunity for manufacturers.
Application Analysis
Chronic Obstructive Pulmonary Disease (COPD) accounts for the highest share in the Non-invasive Ventilation (NIV) masks and circuits market due to its high global prevalence and long-term disease management needs. COPD is a progressive and incurable condition that frequently leads to acute exacerbations and chronic respiratory failure, making non-invasive ventilation a standard and well-established treatment option. NIV is widely used in COPD patients to improve oxygenation, reduce carbon dioxide retention, and decrease the work of breathing, especially during acute flare-ups and post-hospital discharge care. Unlike short-term respiratory conditions, COPD patients often require repeated or continuous NIV therapy, resulting in sustained demand for masks and circuits. Additionally, strong clinical evidence and guideline recommendations support the use of NIV in both hospital and homecare settings for COPD management. The growing elderly population, rising smoking history, and increasing exposure to air pollution further expand the COPD patient pool, reinforcing its dominant share within the application segment.
End User Analysis
Hospitals account for the highest share in the Non-invasive Ventilation (NIV) masks and circuits market due to their central role in managing acute and severe respiratory conditions. Hospitals are the primary point of care for patients with respiratory failure, COPD exacerbations, pneumonia, post-operative breathing complications, and emergency cases, where NIV is often initiated as a first-line ventilation support. Intensive Care Units (ICUs), emergency departments, and high-dependency wards require a continuous and reliable supply of NIV masks and circuits, leading to high procurement volumes and frequent replacement due to infection control protocols. In addition, hospitals handle a large patient inflow, including critically ill, elderly, and post-surgical patients, which increases overall device utilization rates. The presence of skilled healthcare professionals enables proper patient assessment, mask fitting, and therapy adjustment, further supporting NIV adoption in hospital settings. Moreover, favorable reimbursement policies for inpatient respiratory care, access to advanced ventilation equipment, and strict clinical guidelines reinforce hospitals as the dominant end-use segment in the market.
North America Non-invasive Ventilation Masks and Circuits Market Trends
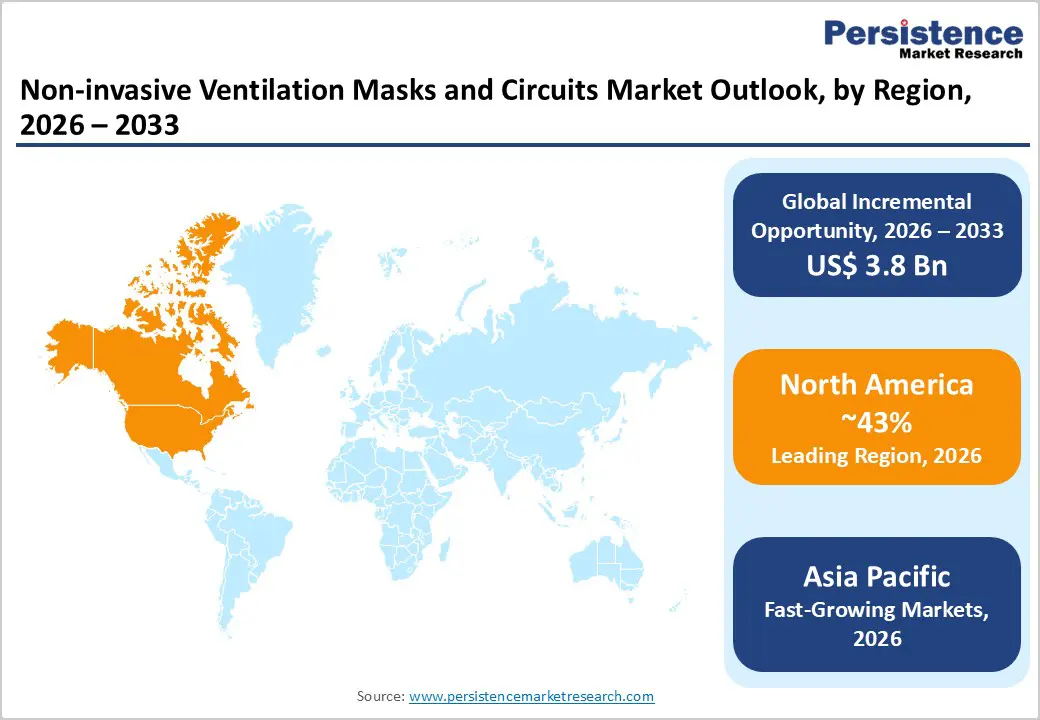
North America represents the leading region in the Non-invasive Ventilation (NIV) masks and circuits market, driven by a combination of high disease burden, advanced healthcare infrastructure, and early adoption of innovative respiratory technologies. The region has a large patient population suffering from COPD, sleep apnea, obesity-related breathing disorders, and acute respiratory failure, which significantly increases demand for NIV therapies across hospital and home care settings. Strong reimbursement frameworks in the U.S. and Canada support the widespread use of non-invasive ventilation in both inpatient and outpatient care, encouraging higher utilization of masks and circuits. In addition, the presence of major medical device manufacturers and continuous product innovations, such as more comfortable mask designs, leak-resistant circuits, and connected NIV systems, further strengthens regional leadership. Growing emphasis on reducing hospital stays, along with rising adoption of home-based ventilation and telemonitoring solutions, is also shaping market trends, reinforcing North America’s dominant position globally.
Asia Pacific Non-invasive Ventilation Masks and Circuits Market Trends and Insights
Asia Pacific is emerging as a high-growth region in the Non-invasive Ventilation (NIV) masks and circuits market, supported by rapid improvements in healthcare access, rising respiratory disease prevalence, and expanding critical care infrastructure. Countries such as China, India, Japan, and South Korea are witnessing increasing cases of COPD, asthma, pneumonia, and sleep-related breathing disorders due to aging populations, air pollution, and lifestyle changes. Growing investments in hospital expansion, ICU capacity, and respiratory care equipment are driving higher adoption of NIV in acute and chronic care settings. In addition, government initiatives aimed at strengthening public healthcare systems and improving emergency and critical care services are supporting market growth. The region is also experiencing rising awareness of non-invasive ventilation as a cost-effective alternative to invasive ventilation, particularly in resource-constrained settings. Furthermore, increasing penetration of homecare services, improving reimbursement coverage, and the entry of local manufacturers offering affordable NIV masks and circuits are accelerating market adoption, positioning Asia Pacific as a key emerging market globally.
Market Structure Analysis
The competitive landscape of the Non-invasive Ventilation (NIV) masks and circuits market is characterized by innovation, product differentiation, and strategic partnerships. Market players are focusing on developing advanced, comfortable, and leak-resistant mask designs along with durable, easy-to-use circuits to meet diverse clinical and homecare needs. Emphasis on research and development has led to integration of smart features and compatibility with a wide range of ventilation platforms. Companies are also expanding their global distribution networks and investing in regulatory approvals to enhance market reach.
Key Market Developments
The global Non-invasive Ventilation Masks and Circuits market is projected to reach approximately US$ 4.1 billion by 2026, reflecting steady growth driven by rising respiratory disease prevalence, technological advancements, and increasing adoption in both hospital and home care settings.
The demand is primarily fueled by the increasing prevalence of chronic respiratory conditions, particularly COPD, which is expected to affect 592 million people by 2050. Additional factors include growing awareness of non-invasive ventilation benefits, technological innovations in masks and circuits, and expansion of homecare services.
Asia Pacific leads the market, accounting for around 43% share in 2025, supported by its large patient population, rising respiratory disease burden, increasing healthcare infrastructure, and growing adoption of NIV therapies.
A significant opportunity lies in homecare integration with telemedicine, as remote monitoring-enabled NIV systems can help reduce hospital readmissions by up to 22%, while improving patient convenience and therapy adherence.
Leading companies driving the market include ResMed, Fisher & Paykel, Philips, and Dräger, all of which focus on product innovation, technological enhancements, and global distribution expansion.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis Units | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
Product
Application
End User
Regions
Middle East & Africa
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author