ID: PMRREP4790| 190 Pages | 7 May 2025 | Format: PDF, Excel, PPT* | Healthcare

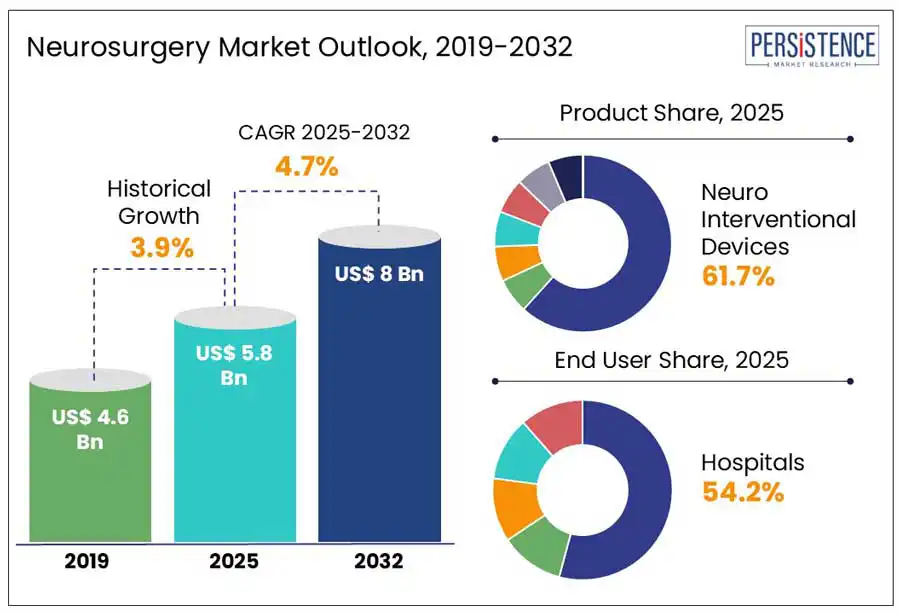
The global neurosurgery market size is anticipated to increase from US$ 5.8 Bn in 2025 to a staggering US$ 8.0 Bn by 2032 a CAGR of 4.7% by 2032. According to the Persistence Market Research report, technological advancements such as AI-assisted navigation, robotics, and minimally invasive techniques makes neurosurgery a popular clinical procedure. Additionally, the rising incidences of neurological disorders, an aging global population, and increasing demand for precision medicine further propel growth. Emerging economies are expanding access to advanced neurosurgical care, while key players invest in innovation and strategic partnerships. From complex brain tumor removals to spinal cord interventions, the field is redefining patient outcomes.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Neurosurgery Market Size (2025E) |
US$ 5.8 Bn |
|
Market Value Forecast (2032F) |
US$ 8.0 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
4.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.9% |
The rising prevalence of neurological disorders is a pivotal driver of the neurosurgery market, creating a sustained demand for advanced surgical interventions. Conditions such as brain tumors, epilepsy, Parkinson’s disease, traumatic brain injuries, and spinal cord disorders are increasing globally due to aging populations, lifestyle changes, and environmental factors. As these conditions often require timely and precise intervention, neurosurgery has become a critical solution for improving patient outcomes and quality of life.
According to the World Health Organization (WHO), over 1 in 3 people are affected by neurological conditions, the leading cause of illness and disability worldwide. Improved diagnostic capabilities and growing awareness have led to earlier detection of neurological issues, thereby increasing the surgical candidate pool.
In low- and middle-income countries (LMIC), previously underdiagnosed cases are now being addressed due to expanding healthcare access and neurology infrastructure. This surge in clinical need is prompting hospitals and surgical centers to invest in modern neurosurgical tools and talent. As a result, the market is witnessing both growth and diversification, with a focus on minimally invasive, high-precision procedures.
Limited access and affordability of neurosurgical care remain significant restraints in the global market, particularly in LMIC. Despite technological advancements, a large chunk of population lacks access to essential neurosurgical services due to geographic, economic, and infrastructural barriers. Many rural and underserved regions do not have equipped facilities or trained specialists, forcing patients to travel long distances, often at unaffordable treatment costs.
Additionally, high procedural and device costs, combined with inconsistent insurance coverage, make advanced neurosurgery inaccessible for many patients. For instance, the cost of surgery, often ranging between US$5,000 to US$15,000, is beyond the reach of most families, especially when not covered under government insurance schemes.
The disparity widens further when public healthcare systems are underfunded or stretched thin. Even in developed nations, out-of-pocket expenses for high-tech procedures such as robotic or image-guided systems can be prohibitive. As a result, many individuals delay or forgo life-saving treatment, directly impacting patient outcomes and limiting the market’s full growth potential. Bridging this affordability and access gap remains a critical challenge.
Technological improvements have resulted in a typical shift away from traditional equipment and toward innovative technology-based equipment that uses less energy. Compared to traditional methods, currently, there are several advanced techniques that make the process of neurosurgery smoother.
The adoption of endoscopic techniques is a great advancement in the market. It requires only smaller incisions as compared to traditional open surgeries, leading to less scarring. The recovery time is also faster. Among others, neurosurgical drills and flexible neuroendoscopes are being developed by companies such as Adeor Medical AG, Stryker Corp, and B. Braun Melsungen AG.
For instance, Elekta, in May 2022, launched Elekta Esprit, which offers significantly faster-automated treatment planning for clinicians and more personalized and patient-friendly treatments. Esprit also offers clinicians superior visualization, as well as remote accessibility and collaboration tools for the treatment team. High adoption rates of new technologies are anticipated to fuel market expansion throughout the projection period.
Neuro interventional devices are leading the neurosurgery product segment due to their pivotal role in minimally invasive treatments of complex cerebrovascular conditions like aneurysms, stroke, and arteriovenous malformations. These devices, such as stents, coils, and catheters, offer faster recovery, reduced surgical trauma, and lower risk compared to open procedures.
The global rise in ischemic strokes and demand for real-time, image-guided precision boosts their adoption. Technological innovations, such as flow diverters and detachable coils, further enhance their effectiveness. As healthcare shifts toward efficiency and patient-centric care, neuro interventional solutions are becoming the gold standard, driving the global neurosurgery market dominance through clinical success and growing procedural volumes.
Traumatic Brain Injuries (TBI) dominate the global market due to their high global prevalence and urgent need for surgical intervention. TBIs commonly result from road accidents, falls, sports injuries, and violence, making them a leading cause of neurological emergencies across all age groups. The unpredictable and acute nature of TBIs often necessitates immediate surgical procedures such as decompressive craniectomy or hematoma evacuation, driving constant demand for neurosurgical services.
Moreover, the increasing use of advanced imaging technologies enables quicker diagnosis and timely treatment, improving outcomes and encouraging surgical intervention. This consistent, high-volume need positions TBI as the dominant segment within the neurosurgery market.
Hospitals dominate the neurosurgery market as a leading end-user segment due to their comprehensive infrastructure, specialized personnel, and advanced diagnostic and surgical equipment. These institutions serve as primary centers for complex neurological procedures, offering 24/7 emergency care, post-operative monitoring, and multidisciplinary collaboration.
The increasing prevalence of neurological disorders drives patient inflow to hospitals, where neurosurgeons can perform intricate operations with cutting-edge technology. Additionally, hospitals often participate in clinical research and innovation, further solidifying their role in neurosurgical advancements. Their capacity to manage high-risk, high-precision surgeries makes them indispensable in delivering superior outcomes in the neurosurgical treatment landscape.
The North American neurosurgery market is experiencing steady growth, driven by rising incidences of neurological disorders, increased adoption of minimally invasive surgical techniques, and advancements in neuroimaging and navigation technologies.
The U.S. holds the largest market share due to robust healthcare infrastructure, significant R&D investment, and widespread use of advanced neurosurgical devices. According to a recent study, as of 2023, the U.S. has approximately 3,800 board-certified neurosurgeons, equating to roughly one neurosurgeon for every 90,000 people. Regulatory support and a strong presence of key market players further fuel regional expansion.
The neurosurgery market in Europe is evolving rapidly, fueled by technological innovation and increasing neurological disorder cases linked to an aging demographic. Western European nations such as Germany, Italy, and the UK are at the forefront, benefiting from well-established healthcare infrastructures and a high concentration of skilled neurosurgeons.
In January 2025, Robeauté, a medtech startup based in Paris specializing in neurosurgical microrobots, completed a €27.2 million funding round to further develop its microbot technology. The company is preparing for human trials planned for 2026 and establish operations in the U.S. as part of its pursuit of FDA approval.
There is also a notable push towards digital health integration, including AI-driven diagnostics and image-guided surgical systems. Additionally, Europe’s focus on training and education in neurosurgical techniques is contributing to improved procedural outcomes.
Asia Pacific is rapidly emerging, with countries such as China, India, Japan, and South Korea leading the way in adopting advanced neurosurgical techniques. India has witnessed a rise in private healthcare facilities offering state-of-the-art neurosurgical services, while South Korea’s emphasis on medical innovation and high-quality care is attracting international patients for neurosurgery.
Additionally, the growing adoption of minimally invasive surgeries, robotic-assisted systems, and advanced imaging technologies is reshaping the landscape. These factors are positioning Asia Pacific as a key emerging market.
The neurosurgery market is highly competitive, with a mix of established global players and emerging startups driving innovation. Companies compete by offering advanced surgical technologies, including minimally invasive procedures, robotic-assisted systems, and cutting-edge imaging solutions. The focus on improving patient outcomes, reducing recovery times, and enhancing precision is central to market strategies.
The global neurosurgery market is estimated to increase from US$ 5.8 Bn in 2025 to US$ 8.0 Bn in 2032.
Innovations in minimally invasive surgical techniques, robotic-assisted surgery, and neuroimaging tools are making neurosurgical procedures safer, more precise, and more effective.
The market is projected to record a CAGR of 4.7% during the forecast period from 2025 to 2032.
The neurosurgery market offers opportunities through technological advancements like AI, robotics, and minimally invasive tools. Expanding healthcare access in emerging markets, rising demand for personalized treatments, and innovations in virtual reality and telemedicine further drive growth.
B. Braun Melsungen AG, Medtronic, DePuy Synthes (Johnson & Johnson Services, Inc), Boston Scientific Corporation, and Stryker are the key players within this market space.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn, Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Indication
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author