ID: PMRREP33149| 214 Pages | 27 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

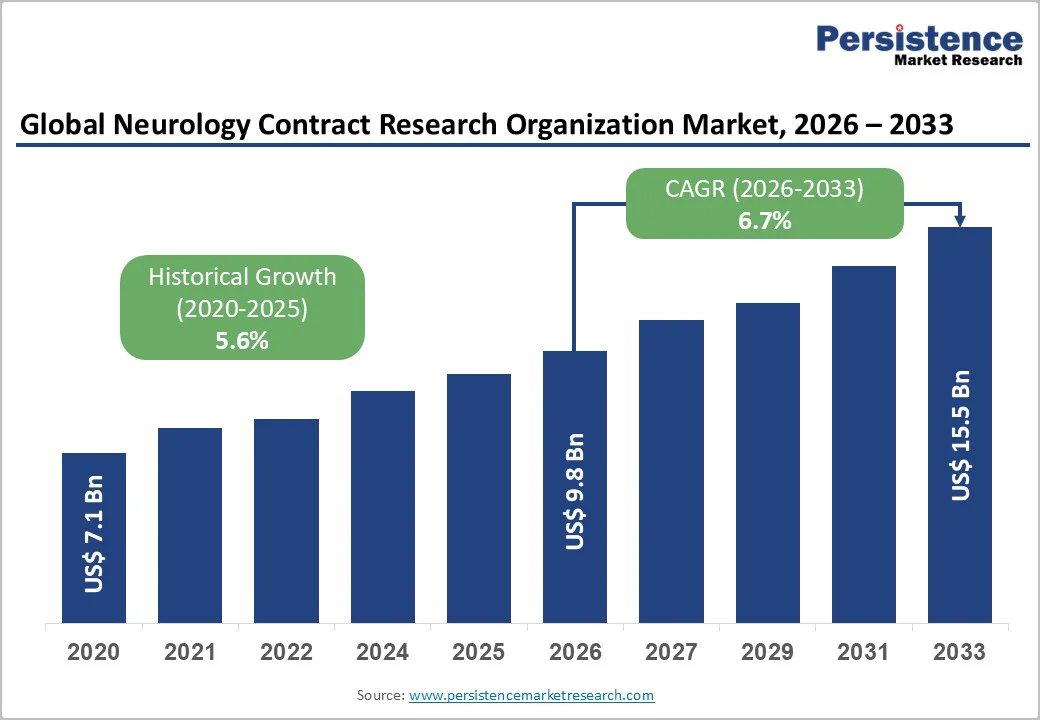
The global neurology contract research organization market size is likely to be valued at US$9.8 Billion in 2026 and is expected to reach US$15.5 Billion by 2033, growing at a CAGR of 6.7% during the forecast period from 2026 to 2033, driven by rising neuroscience R&D investments, greater complexity in clinical trials, and growing demand for outsourced neurological drug development services.
Increased prevalence of neurodegenerative disorders and accelerated regulatory pathways for innovative therapies influence market expansion. The shift toward decentralized trials and biomarker-driven study designs strengthens contract research organization (CRO) engagement across all development phases.
| Key Insights | Details |
|---|---|
|
Neurology Contract Research Organization Market Size (2026E) |
US$9.8 Bn |
|
Market Value Forecast (2033F) |
US$15.5 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
6.7% |
|
Historical Market Growth (CAGR 2020 to 2025) |
5.6% |
The rising incidence of conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, epilepsy, and rare neurodegenerative disorders increases demand for focused clinical research support. Global disease burden projections indicate a steady rise in patient populations, raising the volume of clinical trials and post-marketing studies required across major markets.
Neurology trials often require specialized imaging, complex biomarkers, extended follow-up, and multidisciplinary data integration, which strengthens the role of CROs equipped with neurology-specific expertise. The increasing pressure on pharmaceutical companies to accelerate time-to-market creates sustained demand for outsourced operational capacity, improving long-term market prospects.
Adoption of digital biomarkers, neuroimaging techniques, AI-driven patient monitoring, and electrophysiological assessment tools transforms the structure of neurology trials. These technologies require technical capabilities that many sponsors lack internally, driving greater reliance on CROs with validated platforms and integrated data-management systems. Growth in advanced imaging centers and increased use of adaptive trial designs support complex protocol execution. CROs also play a key role in standardizing data from wearable devices and remote diagnostics, strengthening trial accuracy. Rising investments in precision neurology and targeted therapeutics elevate the scope for specialized contract research offerings.
Biotechnology firms continue to outsource a substantial portion of their neurology pipelines due to the operational demands of large trials, limited in-house infrastructure, and cost-containment priorities. Many emerging biotech developers operate with lean organizational structures and depend heavily on CRO partnerships for regulatory submissions, site management, patient recruitment, and safety monitoring. Outsourcing reduces fixed costs, improves scalability, and enables faster initiation of multi-geographic studies. The continued growth of pipeline assets in neuroinflammation, neuroimmunology, and gene therapy increases the need for CROs capable of supporting specialized protocols and long-term follow-up requirements.
Neurology trials often require large patient pools, extended monitoring periods, specialized diagnostic tools, and multidisciplinary investigator teams. These requirements raise trial budgets and extend development timelines. Rising labor costs, limited availability of neurology-focused investigators, and difficulties in patient retention intensify operational pressures on CROs. The financial burden discourages smaller sponsors from pursuing extensive neurological pipelines, creating fluctuations in study volumes. Cost variability in biomarker analysis and imaging further impacts trial planning and operational stability.
Neurological endpoints remain difficult to standardize, and many conditions lack validated biomarkers or early diagnostic criteria. Regulatory authorities require robust clinical evidence, long-term safety data, and stringent protocol designs. These requirements slow trial initiation and increase administrative workload for CROs. Approval pathways for gene-based neurology treatments require specialized compliance infrastructure that all CROs do not fully possess. These constraints increase operational risk and limit the pace at which organizations can expand capacity.
The shift toward digital patient engagement and remote assessment allows CROs to design flexible neurology trials that reduce monitoring burden and improve recruitment efficiency. Remote cognitive assessment tools, wearable neurological monitors, and home-based data-capture systems create new opportunities for service differentiation. CROs with validated digital-trial frameworks can target expanded service lines with measurable commercial potential. The scalability of decentralized models opens opportunities worth an estimated multi-billion-dollar value over the forecast period.
Mid-sized and early-stage biotechnology firms hold a rising share of global neurology pipelines, creating significant outsourcing opportunities for CROs capable of supporting early-stage research, IND preparation, and multicenter trials. Sponsors seek partners that provide integrated services spanning clinical operations, biomarker analysis, and regulatory support. CROs with strong neurology-specific experience can capture a sizable share of service contracts, enabling substantial market expansion.
Advanced imaging modalities such as MRI, PET, and diffusion-tensor imaging require specialized data-processing and centralized assessment capabilities. CROs offering imaging core laboratories, quantitative biomarker analytics, and AI-enabled interpretation platforms can differentiate strongly in the competitive landscape. The integration of multimodal biomarker data creates an opportunity for value-added service lines with high margins and long-term client retention.
Clinical trial management is anticipated to hold the largest share of 47.8% in 2026. Disorders such as Alzheimer’s disease, epilepsy, and multiple sclerosis typically require Phase II and Phase III trials with several hundred to thousands of participants. This drives sustained reliance on CROs offering integrated study design, site-start-up services, monitoring, patient-recruitment support, and regulatory documentation management. Neurology clinical trials also depend on specialized sites with neuroimaging capabilities, cognitive-assessment expertise, and advanced endpoint measurement tools. For example, late-stage Alzheimer’s trials evaluating beta-amyloid clearance or tau-targeting therapies require continual monitoring, detailed safety data capture, and centralized data review. The operational complexity, long timelines, and high-volume data generation associated with these programs ensure that full-service clinical operations remain the primary revenue contributor within neurology CRO services.
Biomarker and imaging analytics services are expected to grow the fastest, driven by increasing use of advanced neuroimaging, digital biomarkers, and quantitative disease-progression tools. These services enable MRI segmentation, PET quantification, volumetric analysis, electrophysiology interpretation, and machine-learning-based outcome prediction. Neurology trials rely heavily on objective markers to confirm therapeutic effects, especially where symptoms are hard to measure clinically. Rising regulatory acceptance of imaging and data-driven endpoints accelerates adoption. Alzheimer’s studies require centralized PET plaque quantification, multiple sclerosis trials depend on MRI lesion mapping, and neuromuscular gene-therapy programs use digital gait and wearable biomarkers.
Neurodegenerative disorders are expected to remain the largest therapeutic focus in clinical research, representing about 51.3% of the market in 2026. Growth is driven by rising cases of Alzheimer’s, Parkinson’s, Huntington’s, and ALS, along with strong investment in disease-modifying therapies such as monoclonal antibodies, small molecules targeting protein misfolding, and next-generation neuroprotective agents. These studies require extensive CRO support due to complex designs, large patient enrollment, and long follow-up periods. Alzheimer’s and Parkinson’s trials also involve advanced imaging, biomarker testing, cognitive or motor assessments, and digital monitoring tools.
Rare neurological disorders are the fastest-growing clinical research segment, driven by expanding gene-therapy pipelines, targeted molecular approaches, and rising orphan-disease funding. Conditions such as spinal muscular atrophy, Duchenne muscular dystrophy, Friedreich’s ataxia, Batten disease, and hereditary neuropathies require high-value, small-cohort studies with genomic profiling, biomarker-based enrollment, and long-term functional assessments. Regulatory incentives, including orphan-drug status and accelerated pathways, further boost activity. AAV gene-therapy trials in SMA and rare epileptic encephalopathies such as Dravet syndrome depend on CRO expertise for rare-patient recruitment, vector safety monitoring, seizure tracking, and extended follow-up.

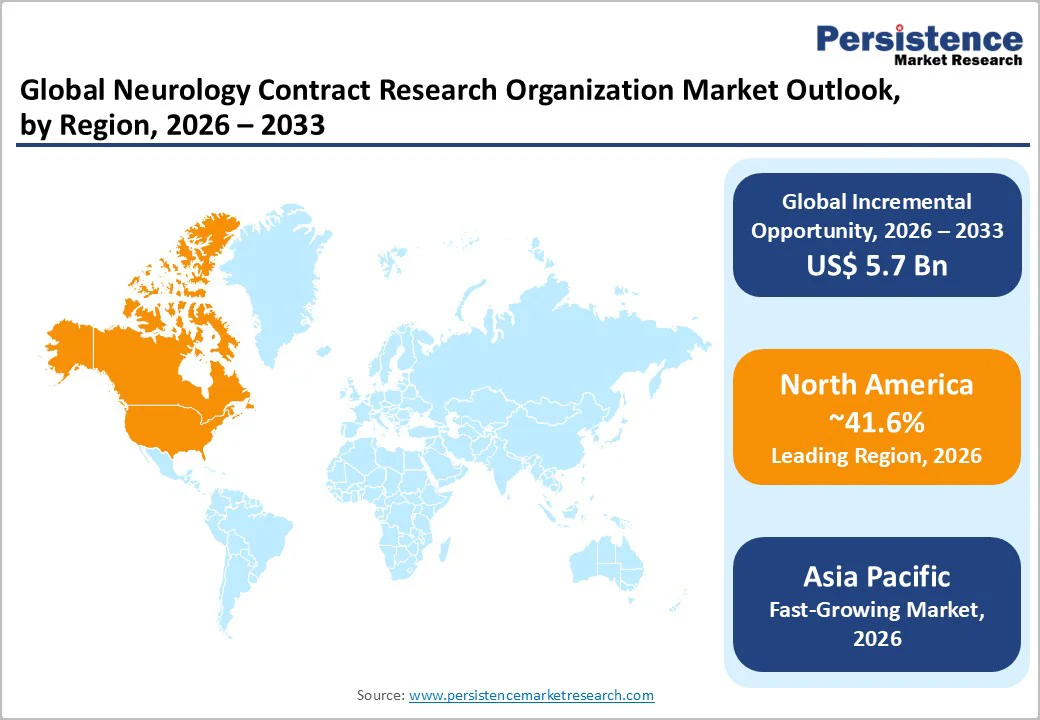
North America is anticipated to hold the largest share of 41.6% in 2026, due to strong clinical research activity, advanced healthcare infrastructure, and significant neurology trial volumes. The U.S. represents the regional leader, supported by extensive biopharmaceutical pipelines and high investments in neurodegenerative and neuroimmunology research. The region benefits from access to specialized investigators, advanced imaging facilities, and extensive patient registries. Growth projections remain steady with continued R&D spending and expansion of precision neurology programs.
Key growth factors include rising Alzheimer’s disease research funding, availability of advanced digital-health platforms, and adoption of decentralized trial frameworks. Regulatory processes encourage innovation while maintaining strict safety standards. The competitive landscape features both global CROs and specialized neurology-focused service providers. Investment activity includes expansions of imaging laboratories, partnerships for digital biomarker development, and new clinical-trial site networks. North America continues to attract substantial early-phase and late-phase neurology studies due to strong operational capabilities.
Europe is driven by strong clinical research activity in major pharmaceutical hubs. Countries such as Germany, the U.K., France, and Spain play key roles due to extensive neurology research programs and established clinical networks. Neuroscience funding continues to grow with strong government and academic participation. The region maintains focus on neurodegenerative disorder trials, rare neurological conditions, and neuroimmunology research.
Regulatory harmonization across the region strengthens trial efficiency and improves patient-recruitment scalability. The competitive environment consists of regional CROs, academic collaborations, and global companies. Europe also benefits from well-developed imaging centers and advanced biomarker laboratories. Investment trends highlight expansions in digital-trial platforms, collaborations between CROs and academic hospitals, and continued development of high-capacity clinical research clusters.
Asia Pacific is projected to be the fastest-growing region with rising neuroscience research activity, growing patient populations, and expanding trial participation. China, Japan, India, and ASEAN countries contribute significantly. Cost-effective trial execution and availability of diverse patient groups strengthen the region’s competitiveness. China experiences rapid growth in neurological clinical-research infrastructure, and Japan maintains strong expertise in neurodegenerative disease trials. India’s expanding clinical-trial ecosystem and increasing number of specialized research centers support regional expansion.
Key growth drivers include improving regulatory frameworks, rising investment in biopharmaceutical innovation, and expansion of clinical-trial site networks. CROs leverage the region’s operational advantages for multi-phase neurology trials. Investment activity centers on clinical-research partnerships, the establishment of biomarker-analysis facilities, and the adoption of digital health tools for remote monitoring. Asia Pacific’s market trajectory remains robust, with strong long-term potential for clinical trial outsourcing.
The global neurology contract research organization market is moderately consolidated, with a mix of global full-service CROs and specialized neurology-focused organizations. Leading players hold a significant share due to established clinical networks, biomarker capabilities, and regulatory expertise. Mid-sized CROs gain momentum through niche neurological expertise and advanced imaging capabilities. Competition focuses on service breadth, data-integration capabilities, therapeutic specialization, and the ability to manage complex neurological protocols across multiple geographic regions.
CROs prioritize innovation, data management integration, and global site-network expansion. Strategic themes include digital-trial enablement, biomarker-driven study design, enhanced imaging analytics, and deeper partnerships with biopharmaceutical developers. Service models emphasize scalability, operational flexibility, and specialized neurological expertise.
The neurology contract research organization market size is estimated to reach US$9.8 Billion in 2026.
By 2033, the neurology contract research organization market value is expected to reach US$15.5 Billion.
Key trends shaping the market include the rising adoption of AI-enabled neuroimaging and digital biomarkers and increased outsourcing of Phase II and Phase III neurology trials.
The clinical trial management services segment leads the market, contributing the largest revenue share due to the complexity of neurology studies, multinational site operations, and high monitoring requirements in Phase II/III programs.
The neurology contract research organization market is projected to grow at a CAGR of 6.7% between 2026 and 2033.
Major players include IQVIA, Labcorp Drug Development, Parexel, ICON plc, and Syneos Health.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Service Type
By Therapeutic Area
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author