ID: PMRREP35324| 192 Pages | 15 May 2025 | Format: PDF, Excel, PPT* | Healthcare

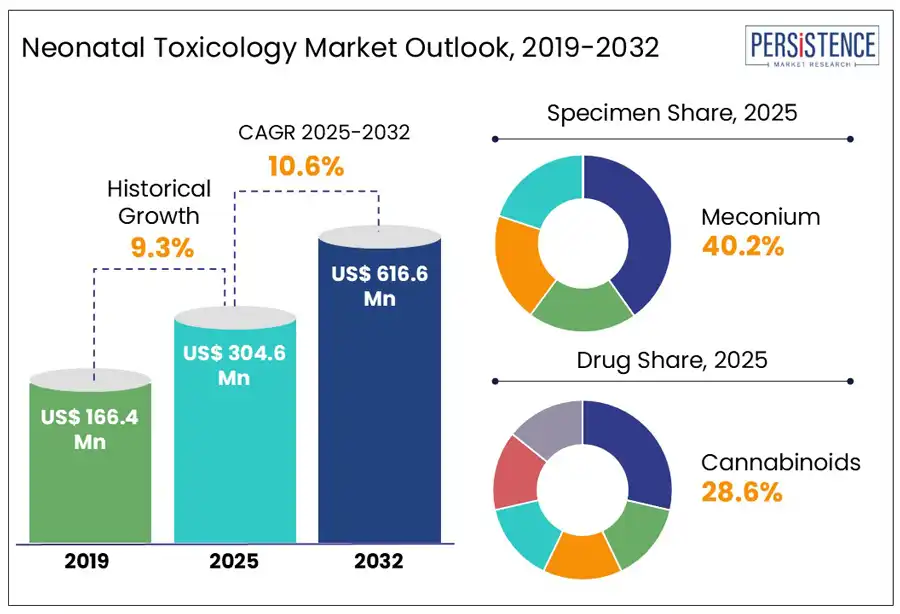
The global neonatal toxicology market size is predicted to reach US$ 616.6 Mn in 2032 from US$ 304.6 Mn in 2025. It will likely witness a CAGR of around 10.6% in the forecast period between 2025 and 2032.
The field of neonatal toxicology has emerged as a significant sub-segment in the broad clinical toxicology and diagnostics landscape. As per a Persistence Market Research report, the market is projected to be propelled by a surge in prenatal substance exposure and the evolution of legal frameworks associated with maternal drug use. Developments in analytical technologies such as multiplex immunoassays and Dried Blood Spot (DBS) testing are expected to help improve detection capabilities.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Neonatal Toxicology Market Size (2025E) |
US$ 304.6 Mn |
|
Market Value Forecast (2032F) |
US$ 616.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
10.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.3% |
Increasing incidence of maternal drug use, specifically the surging misuse of cannabis, stimulants, and opioids during pregnancy, is estimated to spur the neonatal toxicology market growth through 2032. Between 2017 and 2024, more than 1,500 babies were born in Scotland with Neonatal Abstinence Syndrome (NAS), with about 222 cases reported from 2023 to 2024 alone. Most of these cases were concentrated in Grampian, Greater Glasgow, and NHS Lothian. This rise was attributed to budget cuts in alcohol and drug services, pointing to the requirement of well-established toxicology infrastructure in the neonatal infant care market to manage the increasing caseload.
Several diagnostic labs and hospitals worldwide are focusing on investing in innovative neonatal toxicology panels capable of detecting a wide range of substances, including designer drugs and synthetic opioids. They are focusing on developing non-invasive methods such as umbilical cord and meconium testing as unlike conventional urine testing, these offer higher accuracy and a longer detection window. U.S.-based firms such as ARUP Laboratories and Labcorp recently extended their neonatal toxicology lines to include unique panels that can test for more than 100 substances with fast turnaround times.
The potential for legal repercussions associated with positive neonatal toxicology tests is poised to affect testing protocols and clinical workflows. It will also likely shape the willingness of pregnant women to seek prenatal care. In several states across the U.S., a positive toxicology result in a newborn can often lead to mandatory reporting to Child Protective Services (CPS). It further leads to criminal charges, custody loss, or investigations related to the mother. This legal entanglement is expected to push healthcare providers to re-evaluate when and how they conduct toxicology tests. It is predicted to create a tension between legal compliance and clinical care, thereby hampering growth.
Hospitals are hence increasingly demanding legal counsel to develop protocols to balance ethical care with mandatory reporting norms. The University of Michigan Health System, for instance, recently rolled out a revised testing policy. It includes staff training on the legal implications of test results as well as patient consent for non-emergency drug screening. This model has been commended for lowering unnecessary CPS referrals while complying with state laws.
Developments in the mass spectrometry market are anticipated to create new opportunities in the field of neonatal toxicology in the forecast period. Conventional immunoassay-based techniques often lack specificity and sensitivity, mainly for polysubstance exposure and emerging synthetic drugs. These techniques hence result in incomplete screening or false negatives. Innovative techniques such as Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS), however, are capable of accurately quantifying trace levels of more than 100 substances from a single sample. This high precision is specifically important for neonates, where timely diagnosis of exposure can affect life-saving interventions.
The automation and miniaturization of mass spectrometry systems is a significant innovation in this field. It allows for integration into hospital labs with quick turnaround times. In 2023, Thermo Fisher Scientific, for example, launched a fully automated LC-MS/MS platform made for toxicology labs with limited staffing. It processes umbilical cord or meconium samples with minimal manual preparation, thereby lowering analysis time from days to hours. The launch of similar products is estimated to enable real-time decision-making in neonatal ICUs, reducing delays in treatment and reliance on centralized reference labs.
In terms of specimen, the market is trifurcated into urine, umbilical cord, and meconium. Among these, meconium is predicted to generate around 40.2% of the neonatal toxicology market share in 2025. It is considered an ideal specimen owing to its ability to retain a wide array of drug metabolites over an extended gestational period. Unlike blood or urine, which reflects only recent exposure, meconium begins forming around the 12th to 16th week of gestation and accumulates substances till birth. This makes it suitable for detecting repeated or chronic drug exposure throughout the second and third trimesters. Its slow turnover and high lipid content often enable it to trap both parent drugs and their metabolites, delivering an in-depth exposure history that short-term matrices often miss.
Umbilical cord, on the other hand, is expected to witness a considerable CAGR from 2025 to 2032. This is due to its potential to detect a wide spectrum of metabolites and drugs without delaying early intervention. It is usually collected right after delivery, ensuring no loss of diagnostic opportunity. The Journal of Analytical Toxicology mentions that novel LC-MS/MS panels can now screen for more than 60 substances in umbilical cord tissue, including fentanyl and its analogs. It also found that umbilical cord testing detected 94% of confirmed opioid exposures in neonates. Similar innovations are also likely to augment the umbilical cord blood banking market, as both therapeutic and diagnostic applications of cord-derived specimens continue to surge.
Based on drug, the market is segregated into cannabinoids, opioids, cocaine, benzodiazepines, and amphetamines. Out of these, cannabinoids are poised to account for nearly 28.6% share in 2025. These have become the most frequently targeted and detected drug class, backed by their surging prevalence among pregnant women and the issues associated with legalization trends. With the broad recreational use and decriminalization of cannabis across parts of Europe and North America, the perception of its safety during pregnancy has changed.
As per the U.S. Centers for Disease Control and Prevention (CDC), cannabis was the most commonly reported substance among pregnant women, with self-reported usage rates rising from 4.2% in 2018 to 7.1% in 2022. The surge in perinatal cannabis exposure is also driving interest in the cannabinoid biosynthesis market, as diagnostic companies demand standardized biomarkers for enhanced toxicology reference standards.
Opioids, on the other hand, are envisioned to showcase a steady CAGR from 2025 to 2032. These are prioritized because even late-gestation or short-term exposure can lead to withdrawal symptoms in neonates, including respiratory issues, tremors, seizures, and feeding problems. Unlike other drugs, opioid exposure often requires prolonged and immediate postnatal intervention, including extended Neonatal Intensive Care Unit (NICU) stays. A study by Pediatrics International revealed that opioid-exposed neonates had an average hospital stay of 14 days, which was around four times that of non-exposed infants. This represents a significant burden on the healthcare infrastructure, thereby spurring demand for unique toxicology methods.
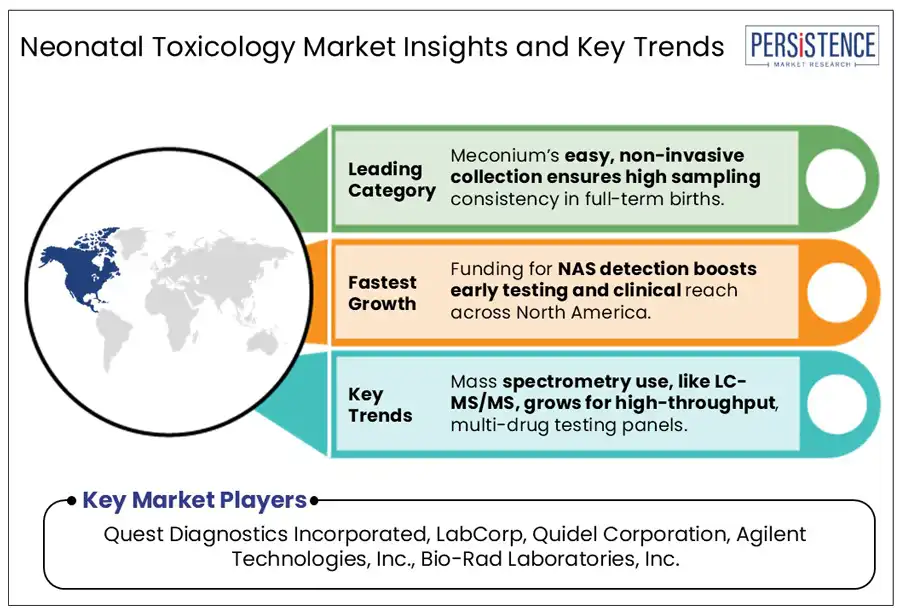
In 2025, North America is speculated to account for a share of about 50.6%. The region is estimated to be spearheaded by the U.S. neonatal toxicology market through 2032. It is attributed to the surging prevalence of polysubstance use among pregnant women, legalization of cannabis, and opioid crisis. As per the Healthcare Cost and Utilization Project (HCUP), there were more than 100,000 hospital stays associated with NAS in 2023 alone in the country. It pointed toward the urgent requirement for innovative toxicology screening in maternity wards. The rapid shift toward standardized or universal screening protocols in the U.S. to address disparities in risk-based testing is expected to open new opportunities.
A recent policy change in Pennsylvania mandated universal toxicology testing for all Medicaid births. The decision was taken after a state health department audit exhibited racial disparities in who was screened and referred for child protective services. This change has not only lowered the likelihood of bias in reporting and intervention but also raised detection rates. Hospitals in Colorado and California, on the other hand, have extended their neonatal screening panels to include a wide range of cannabinoid metabolites. This is attributed to the legalization of recreational cannabis and the shifting perception of its safety during pregnancy.
While some countries in Europe, such as the Netherlands, Germany, and Sweden have well-established neonatal toxicology protocols, others are still striving to keep up due to cultural attitudes toward drug use and differences in healthcare norms. The European Monitoring Center for Drugs and Drug Addiction (EMCDDA) reported that benzodiazepines, opioids, and cannabis were the most detected substances in neonates in 2024, mainly in urban hospitals serving socioeconomically disadvantaged individuals.
Eastern Europe has been experiencing high incidence of opioid misuse in recent years. In parts of the Czech Republic and Romania, for example, NAS cases surged by more than 30% from 2019 to 2024, according to Euro-Peristat. The situation has hence boosted new funding in 2025 under the European Commission’s Healthier Together initiative. It will likely help support non-invasive screening research and early NAS detection activities in these areas. Prague and Bucharest have been seeing the initiation of pilot projects to test Dried Blood Spot (DBS)-based toxicology screening for high-risk births. Such projects are expected to enable affordable scaling across low-resource hospitals.
Asia Pacific is currently evolving with rising investment in maternal-child health infrastructure, increasing awareness of prenatal substance exposure, and shifting drug use patterns. Countries such as South Korea, Australia, and Japan have relatively robust toxicological screening protocols. Other countries, including the Philippines, Indonesia, and India, however, are still at the nascent stage of integrating neonatal toxicology into routine neonatal care. The Asia Pacific Association of Medical Toxicology (APAMT) mentioned that only 40% of surveyed hospitals across the region conducted any form of routine neonatal drug screening in 2023.
A key challenge in Asia Pacific is the increasing cases of maternal substance use in certain pockets of the region. The National Drug Strategy Household Survey 2022, for example, found that nearly 5% of pregnant women in Australia reported using illicit drugs, with opioids and cannabis being the most common. Similar findings compelled hospitals in the country to embrace risk-based or universal neonatal toxicology testing. Another study published in 2024 in the Lancet Regional Health revealed a 3.1% prevalence of neonatal exposure to methamphetamine in specific parts of the Philippines. It was attributed to the lack of prenatal screening and ongoing meth epidemic. There will likely be a significant call for more structured protocols in this field across Asia Pacific in the near future.
The neonatal toxicology market houses various emerging biotechnology companies, specialized laboratories, and well-established diagnostic giants. Leading players are focusing on leveraging their innovative testing technologies and extensive laboratory networks to remain at the forefront of growth. They are providing comprehensive toxicology panels capable of detecting a wide array of substances. Strategic acquisitions and collaborations are also transforming the competitive landscape. Key players are acquiring small-scale companies to integrate innovative genetic testing technologies into their existing neonatal screening solutions. Such steps are helping them to come up with enhanced product lines for consumers.
The market is projected to reach US$ 304.6 Mn in 2025.
Increasing drug abuse among pregnant women and constant evolution of legal frameworks are the key market drivers.
The market is poised to witness a CAGR of 10.6% from 2025 to 2032.
Surging automation of testing methods and increasing desire for non-invasive techniques are the key market opportunities.
Quest Diagnostics Incorporated, LabCorp, and Quidel Corporation are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Specimen
By Technology
By Drug
By End Use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author