ID: PMRREP35181| 182 Pages | 28 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

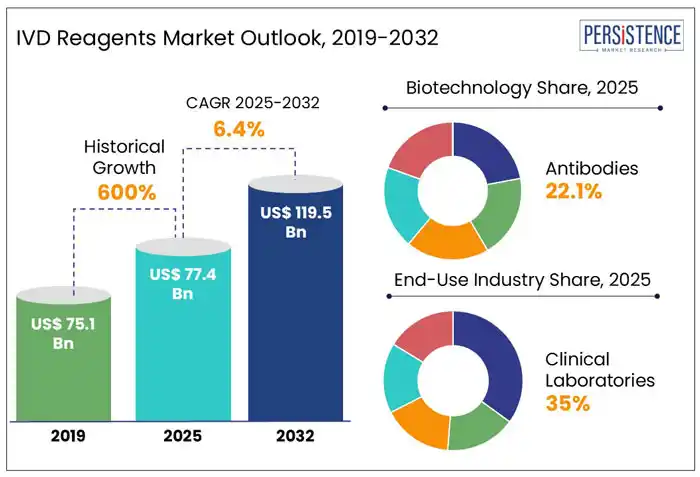
The global IVD reagents market size is estimated to reach US$ 77.4 Bn in 2025. It is likely to witness a CAGR of 6.4% from 2025 to 2032 and attain a value of US$ 119.5 Bn by 2032. Factors such as growing demand for personalized medicines and biomarker-based tests, booming biotech sector, technological innovations in molecular diagnostics, and increasing prevalence of chronic diseases globally are likely to surge adoption for IVD reagents through 2032. In vitro diagnostic (IVD) reagents are chemicals or substances used in diagnostic tests performed outside the body (in vitro) to detect, measure, or react with components of a sample, aiding in disease diagnosis, monitoring, and treatment.
Key Industry Highlights:
| Global Market Attribute | Key Insights |
| IVD Reagents Market Size (2025E) | US$ 77.4 Bn |
| Market Value Forecast (2032F) | US$ 119.5 Bn |
| Projected Growth (CAGR 2025 to 2032) | 6.4% |
| Historical Market Growth Rate (CAGR 2019 to 2024) | 6% |
Automated analyzers are significantly contributing to the expansion of the IVD reagents market by enhancing efficiency and accuracy in diagnostic workflows. They automate multi-test sample analysis, increasing throughput and reducing turnaround times, which in turn boosts the demand for IVD reagents as they process high volumes of samples. The integration of automated analyzers with point-of-care testing systems further expands their reach beyond traditional laboratories, increasing the demand for specialized reagents.
Their adoption in emerging economies supports market growth by creating new opportunities for both automated analyzers and associated IVD reagents. Continuous technological advancements in these systems are further likely to drive innovation in reagent development, encouraging the adoption of advanced diagnostic tools and fueling market expansion during the forecast period.
The market for IVD reagents is heavily regulated to ensure safety, efficacy, and quality of diagnostic procedures. These regulations vary by country but generally involve a classification system based on risk levels. For instance, in China, IVD reagents are classified into three classes, with Class I being subject to filing and Classes II and III requiring registration with the National Medical Products Administration (NMPA). In the U.S., the FDA regulates IVDs as medical devices, requiring compliance with labeling requirements under 21 CFR 809 and submission of studies confirming their safety and effectiveness.
Similarly, in India, IVDs are regulated under the Medical Device Rules, 2017, by the Central Drugs Standard Control Organization (CDSCO), necessitating licenses for manufacturing, importing, and selling. Manufacturers need to adhere to the evolving regulations and standards to avoid non-compliance issues. Such regulations are likely to create problems for small and medium-sized enterprises due to their associated costs and complexity, leading to restricted market growth to a certain extent.
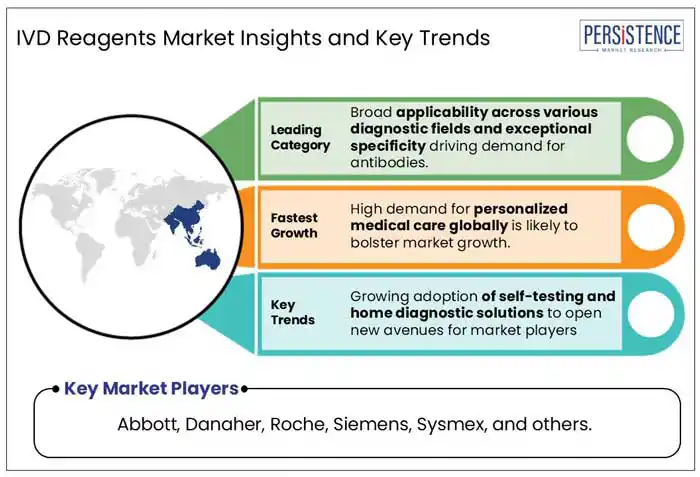
High adoption of self-testing and home diagnostic procedures is anticipated to open new growth avenues for companies operating in the market for IVD reagents. This trend is driven by the increasing demand for delivering care closer to patients, which has been accelerated by the COVID-19 pandemic.
The expansion of point-of-care testing (POCT) reagents has significantly enhanced the availability of tests that can be conducted outside traditional laboratory settings. For instance, immunoassay-based POC testing analyzers offer high sensitivity and patient compliance, reducing the time needed to diagnose and manage diseases such as HIV, malaria, and tuberculosis. As a result, POC testing products are becoming more widely used to support informed clinical decisions and improve healthcare outcomes.
The integration of advanced technologies such as AI and IoT is further expected to play an important role in enhancing diagnostic capabilities, presenting exciting growth opportunities for market players to strengthen their positions.
Based on the type of reagents, the antibodies segment is expected to hold a share of about 23.5% in 2025. Antibodies are increasingly being used as IVD reagents due to their exceptional specificity, sensitivity, and broad applicability across various diagnostic fields. Antibodies play a crucial role in immunoassays, molecular diagnostics, and point-of-care (POC) tests, enabling accurate illness identification.
Antibody-based assays such as Enzyme-Linked Immunosorbent Assay (ELISA), lateral flow assays, and chemiluminescence immunoassays are frequently used in the diagnosis of infectious diseases. Rapid antigen and serological testing became more and more necessary as a result of the COVID-19 pandemic, highlighting the role that antibodies play in point-of-care testing and extensive disease surveillance. Antibodies play a critical role in oncology by identifying tumor markers, including CA-125 (ovarian cancer), PSA (prostate cancer), and HER2 (breast cancer), allowing for early diagnosis and therapy effectiveness monitoring.
Clinical Laboratories Segment to Lead Drive by Enhanced Accuracy and Regulatory Compliance
Based on end use industry, the clinical laboratories segment is forecasted to hold about 35% of the market share in 2025. Clinical labs are the preferred choice for highly accurate and validated diagnostic testing due to their advanced infrastructure, stringent quality control measures, and expertise in handling complex tests.
One of the fundamental benefits of clinical laboratories is their capacity to perform high-throughput testing, processing huge numbers of samples efficiently while adhering to regulatory standards. This establishes them as the gold standard for confirmatory testing, particularly in crucial areas such as oncology, infectious diseases, and autoimmune diagnostics.
North America is anticipated to dominate the market for IVD reagents with a share of about 38.7% in 2025. The region’s strong healthcare infrastructure and ongoing investments in diagnostic technologies are factors supporting the market growth during the forecast period.
According to the American Hospital Association (AHA), as of 2025, there are 6,093 registered hospitals and 913,136 beds in the U.S. The presence of well-established healthcare infrastructure significantly boosts the demand for IVD reagents by enhancing diagnostic capabilities, increasing accessibility to care, and ensuring regulatory compliance. In addition, robust healthcare systems often receive government support and funding, leading to increasing investments in cutting-edge technologies such as point-of-care testing (POCT) and digital diagnostics.
For example, in February 2025, the National Institute of Allergy and Infectious Diseases (NIAID) announced the launch of its program that offers platform testing, planning, designing, and reagent support to accelerate product development of in vitro diagnostics (IVD) for infectious diseases, from research feasibility through clinical validation. This program is aimed at supporting in vitro diagnostics (IVD) companies in the U.S.
The global IVD reagents market in Asia Pacific is projected to witness substantial growth in the forthcoming years. The growth is attributable to the presence of established in vitro diagnostic (IVD) companies such as Sysmex Corporation, Fujifilm, Eiken Chemical Co., Ltd, Hangzhou AllTest Biotech Co., Ltd, Shimadzu Corporation, and others. These companies are contributing to the growth of the market by expanding their market presence through strategic partnerships and investments in research and development activities. For instance,
Eiken Chemical has been adopting its Loop-mediated Isothermal Amplification (LAMP) technology for rapid genetic testing. This technology is used for detecting diseases like tuberculosis, malaria, and COVID-19. The company continues to focus on research and development activities, with about 150 staff dedicated to developing new technologies and products.
The IVD reagents market in Europe is likely to register significant growth in the forthcoming years. Factors such as the presence of stringent regulatory compliance, increasing adoption of molecular diagnostic testing in personalized medicine, and expansion of testing capabilities in clinical laboratories are projected to drive market growth between 2025 and 2032. The implementation of the In Vitro Diagnostic Regulation (IVDR) in Europe has established stringent requirements for diagnostic products, prompting major companies such as Roche Diagnostics, Abbott Laboratories, Thermo Fischer Scientific, and others to invest in research and development to meet the diagnostic standards.
The global IVD reagents market is driven by ongoing developments in automation, immunoassays, and molecular diagnostics. Businesses are making significant investments in next-generation sequencing (NGS) technologies, point-of-care (POC) solutions, and AI-powered platforms. Strategic collaborations, mergers, and acquisitions are common as industry players seek to expand their product offerings and geographic reach. Additionally, companies are focusing on gaining a stronghold in the market by manufacturing quick, easy-to-use diagnostic reagents on account of rapid growth in the telemedicine vertical of the healthcare industry.
The IVD Reagents market size is US$ 77.4 Bn in 2025.
The industry will likely be valued at US$ 119.5 Bn in 2032.
A few of the leading players in the market are Abbott, Beacon, Thermo Fisher Scientific, Danaher, and Siemens.
The industry is estimated to rise at a CAGR of 6.4% through 2032.
North America is projected to hold the largest share of the industry in 2025 as well as through the forecast period.
| Report Attribute | Details |
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
| Customization and Pricing | Available upon request |
By Type of Reagent
By Technology
By Application
By End-Use Industry
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author