ID: PMRREP3273| 230 Pages | 9 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

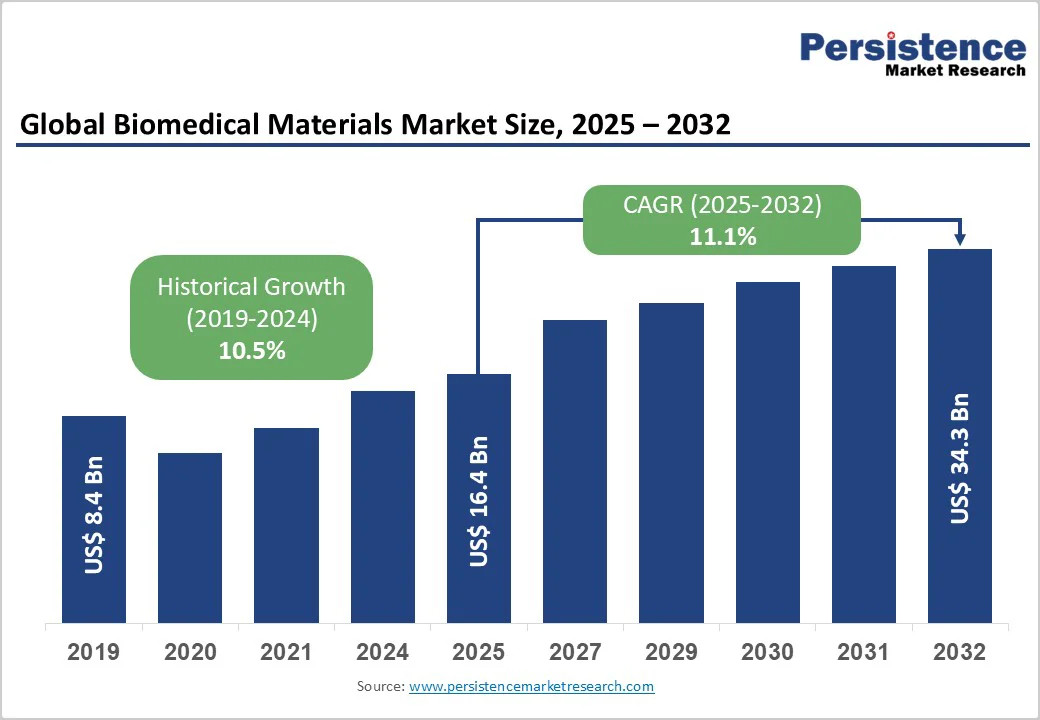
The global biomedical materials market size is likely to be valued US$16.4 Billion in 2025, expected to reach US$34.3 Billion by 2032, with achieving at a CAGR of 11.1% during the forecast period from 2025 to 2032 driven by increasing demand for advanced medical implants, rising prevalence of chronic diseases, and advancements in regenerative medicine.
The market is further propelled by innovations in 3D-printed biomaterials and bioactive ceramics, catering to preferences for personalised and durable medical solutions. The growing acceptance of medical materials in minimally invasive procedures and regenerative therapies is a key growth factor.
| Key Insights | Details |
|---|---|
|
Biomedical Materials Market Size (2025E) |
US$16.4 Bn |
|
Market Value Forecast (2032F) |
US$34.3 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
11.1% |
|
Historical Market Growth (CAGR 2019 to 2024) |
10.5% |
The increasing demand for medical implants and regenerative medicine is a primary driver of the biomedical materials market. Increasing incidences of orthopaedic disorders, cardiovascular diseases, dental issues, and trauma-related injuries have fueled the need for implants such as joint replacements, dental prosthetics, and cardiovascular stents. Ageing populations in developed regions, combined with lifestyle-related health conditions, are further escalating the demand for durable, biocompatible, and long-lasting implants that improve patient outcomes and quality of life.
Simultaneously, regenerative medicine is gaining momentum as a transformative approach to repairing or replacing damaged tissues and organs. Healthcare materials such as natural and synthetic polymers, hydrogels, bioactive ceramics, and metallic alloys are being used to create scaffolds, matrices, and bioengineered constructs that support cell growth, tissue regeneration, and functional restoration. Advances in 3D and 4D printing technologies have further enabled patient-specific implants and tissue constructs, enhancing surgical precision and treatment efficacy.
The high costs of advanced biomaterials and stringent regulatory requirements pose significant restraints on market growth. Developing advanced biomaterials, such as biocompatible polymers, bioresorbable scaffolds, and metallic implants, requires substantial investment in research and development, including preclinical and clinical testing to ensure safety and efficacy. The cost of sourcing high-quality raw materials, manufacturing under sterile and controlled conditions, and implementing advanced technologies such as 3D printing further adds to overall expenses.
Regulatory frameworks across regions impose rigorous approval processes. Agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other national authorities require extensive documentation, clinical trials, and compliance with biocompatibility, mechanical, and sterilisation standards. These approvals are essential to ensure patient safety, especially for implants and devices that interact directly with human tissue. Stringent regulations can delay product launches and increase development timelines, creating entry barriers for new players. High costs and compliance requirements also impact pricing strategies, limiting accessibility in emerging markets.
Expansion in 3D-printed biomaterials and tissue engineering presents significant growth opportunities for the biomedical materials market. 3D printing technologies enable the fabrication of complex, customised implants, prosthetics, and scaffolds with high precision, tailored to individual anatomical requirements. This advancement allows surgeons to plan and execute procedures more effectively, reducing surgical risks and improving patient outcomes. Additionally, 3D-printed scaffolds made from biocompatible and bioresorbable materials are increasingly used in tissue engineering, supporting cell growth and tissue regeneration for applications in orthopaedics, cardiovascular repair, and reconstructive surgery.
Tissue engineering itself is gaining momentum as it addresses the limitations of traditional organ transplantation and synthetic implants. Regenerative materials such as natural polymers, hydrogels, and bioactive ceramics are used to create three-dimensional constructs that mimic the extracellular matrix, promoting tissue repair and regeneration. Ongoing research focuses on enhancing mechanical strength, biodegradability, and biological functionality, enabling the development of complex tissues and even organoids.
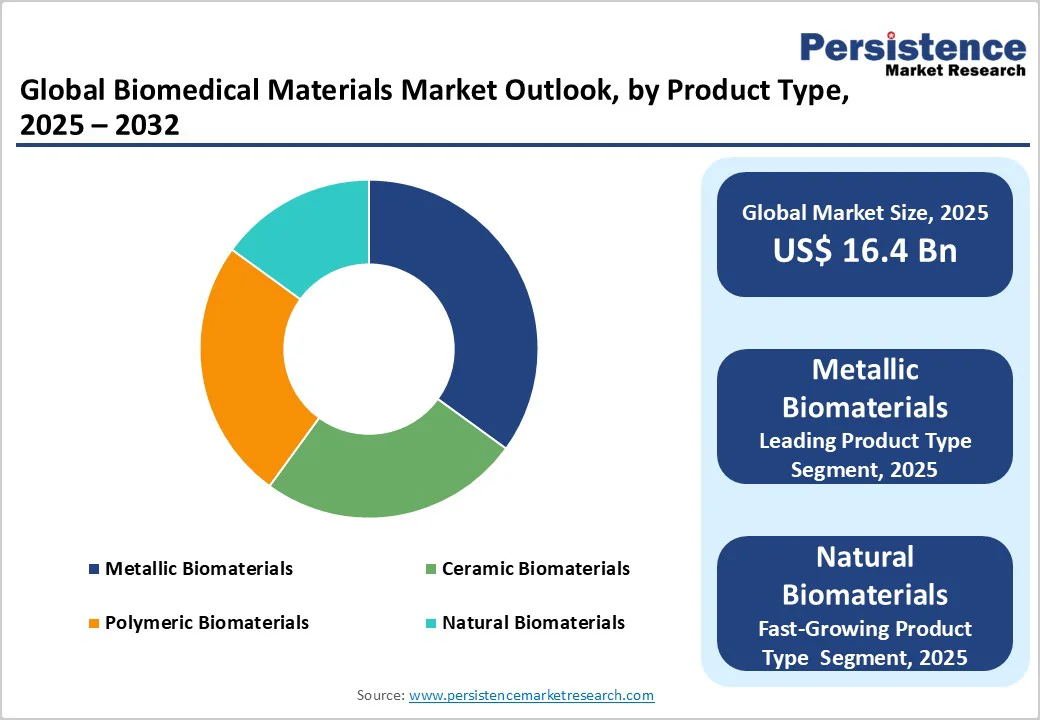
Metallic biomaterials dominate the market, accounting for 40% of the share in 2025. Its prevalence is driven by exceptional strength, durability, and corrosion resistance, making metals like titanium and stainless-steel ideal for orthopaedic implants, joint replacements, and cardiovascular devices. These properties ensure long-lasting performance and reliability in critical medical applications.
Natural biomaterials are the fastest-growing segment, driven by increasing demand for bioresorbable and biocompatible materials in tissue engineering and regenerative medicine. Derived from natural sources such as collagen, chitosan, and alginate, these materials support cell growth, minimise immune response, and degrade safely in the body, making them ideal for advanced medical and implantable applications.
Orthopaedic leads with a 30% share, driven by the increasing number of joint replacement surgeries and musculoskeletal treatments. Ageing populations, coupled with rising incidences of arthritis and bone-related disorders, are fueling demand for implants and prosthetics. High-performance, biocompatible materials ensure durability, safety, and improved patient outcomes, sustaining market dominance in this segment.
Wound Healing is the fastest-growing, fueled using bioactive ceramics, hydrogels, and advanced polymer scaffolds. These materials promote tissue regeneration, accelerate healing, and manage chronic wounds effectively. The increasing prevalence of diabetes, pressure ulcers, and surgical wounds is driving adoption in hospitals and specialised care centres globally.
Healthcare Facilities hold a 50% share. Hospitals, clinics, and specialised medical centres extensively use healthcare materials in surgical implants, prosthetics, and orthopaedic devices. The high adoption is driven by the need for durable, biocompatible, and safe materials that enhance patient outcomes, support complex surgeries, and improve the effectiveness of medical interventions.
Tissue Engineering is the fastest-growing field, driven by increasing demand for regenerative therapies that repair or replace damaged organs and tissues. Advances in biomaterials, stem cell research, and 3D bioprinting are enabling the development of functional tissue constructs, offering innovative solutions for organ regeneration and personalised medical treatments.
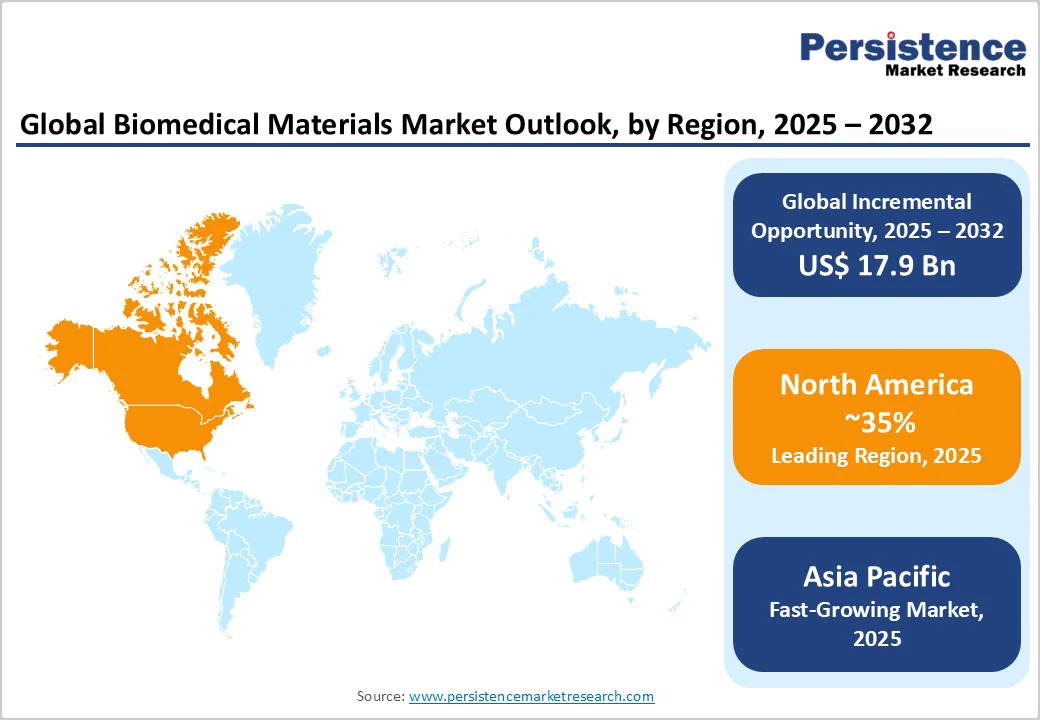
North America accounts for 35% in 2025, driven primarily by high healthcare spending, advanced infrastructure, and substantial research and development investments. The United States, in particular, benefits from a combination of strong private and public healthcare funding, cutting-edge medical technologies, and the presence of major market players such as Medtronic, Stryker, and Zimmer Biomet. These companies invest heavily in R&D to develop innovative, biocompatible materials for applications in orthopaedics, cardiovascular devices, dental implants, and minimally invasive surgical tools.
The region also experiences high adoption of advanced biomaterials due to growing demand for patient-specific implants, regenerative medicine solutions, and smart polymer technologies. Insurance coverage and reimbursement policies further support the adoption of premium medical materials, enabling hospitals and clinics to integrate novel solutions into patient care.
Europe holds about 30% market share, led by Germany and France, emerging as leading contributors. The region benefits from robust medical device industries, advanced healthcare infrastructure, and strong regulatory frameworks that prioritise patient safety and product quality. European governments and institutions actively support innovation through funding programs such as EU Horizon 2020, which provide financial backing for research in biocompatible and sustainable materials. This funding accelerates the development of next-generation biomaterials for applications in orthopaedics, cardiovascular devices, dental implants, and tissue engineering.
Germany’s well-established engineering and healthcare sectors, coupled with its high adoption of advanced medical technologies, drive demand for high-performance medical materials. France similarly invests heavily in R&D and healthcare innovation, enabling manufacturers to design and commercialise cutting-edge solutions tailored to clinical needs.
Asia Pacific commands around a 25% share and is the fastest-growing region, driven by rapid developments in healthcare infrastructure and rising medical device demand. China’s expanding medical device industry, fueled by government initiatives to enhance healthcare access, technological advancements, and increasing investment in hospitals and diagnostic centres, is a major growth catalyst. The country is witnessing a surge in procedures such as orthopaedic surgeries, cardiovascular interventions, and dental implants, all of which require high-quality biomedical materials.
India’s ageing population is creating a heightened need for affordable and accessible healthcare solutions, particularly in orthopaedics, cardiovascular care, and prosthetics. The growing prevalence of chronic diseases and the rising middle-class population further boost demand for cost-effective implants and biomaterials.
The global biomedical materials market is highly competitive, driven by rapid advancements in healthcare, increasing demand for medical devices, and rising patient awareness of innovative treatment options. Leading companies such as Corbion NV, BASF SE, DuPont, Covestro AG, and Medtronic are investing heavily in research and development to create advanced biomaterials that are biocompatible, durable, and suitable for diverse medical applications, including orthopaedics, cardiovascular devices, dental implants, and drug delivery systems.
Strategic acquisitions and partnerships are common, allowing firms to expand their product portfolios, enter new geographic markets, and access cutting-edge technologies. Sustainable and eco-friendly material innovations are gaining prominence, as stakeholders prioritise materials that are recyclable, non-toxic, and derived from renewable sources.
The global biomedical materials market is projected to reach US$16.4 Bn in 2025, driven by demand for advanced implants and regenerative therapies.
Use of biomaterials for scaffolds, organ repair, and bioengineered tissues fuels market expansion.
The market is poised to witness a CAGR of 11.1% from 2025 to 2032, supported by 3D printing and tissue engineering advancements.
Expansion in tissue engineering with natural biomaterials offers opportunities for regenerative organ repair solutions.
Zimmer Biomet, Medtronic, Stryker, Corbion NV, and BASF SE lead through innovations in biomedical materials for implants and tissue engineering.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Application
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author