ID: PMRREP35610| 178 Pages | 12 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

The global antibiotics market size is likely to be valued at US$ 50.7 Bn in 2025, and is expected to reach US$ 70.3 Bn by 2032, growing at a CAGR of 4.8% during the forecast period 2025 - 2032.
Key Industry Highlights:
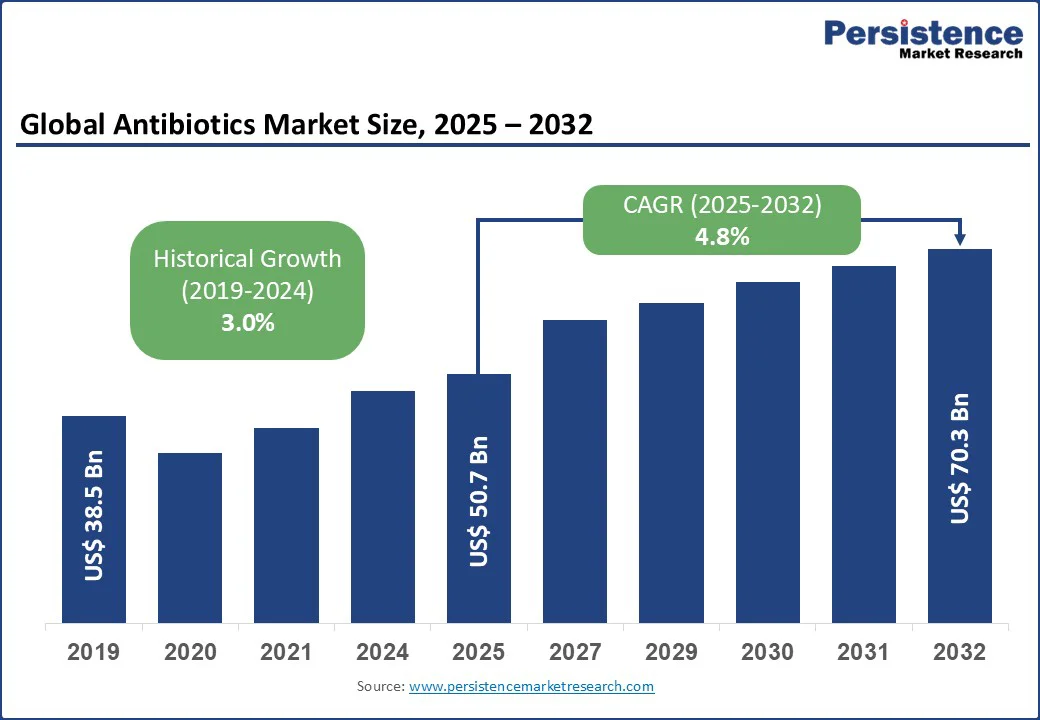
| Global Market Attribute | Key Insights |
|---|---|
| Antibiotics Market Size (2025E) | US$ 50.7 Bn |
| Market Value Forecast (2032F) | US$ 70.3 Bn |
| Projected Growth (CAGR 2025 to 2032) | 4.8% |
| Historical Market Growth (CAGR 2019 to 2024) | 3.0% |
Stringent global policies for combating antibiotic misuse, proactive government initiatives fostering antibiotic stewardship, and substantial investments in R&D for effective antibiotics against resistant strains are the prime growth stimulants.
The accelerated innovation in novel and broad-spectrum antibiotics fueled by the mounting crisis of antimicrobial resistance (AMR) is the foremost driving factor for this market. Unlike traditional antibiotics facing diminishing efficacy due to resistant bacterial strains, breakthroughs in synthetic biology, AI-powered drug discovery, and precision fermentation are enabling the rapid development of next-generation antibiotics with enhanced safety and targeted action.
For instance, in August 2025, researchers at the Massachusetts Institute of Technology (MIT) leveraged generative deep learning to design entirely novel antibiotic compounds, screening over 36 million computationally generated candidates, and successfully identified two lead molecules, NG1 and DN1.
These molecules effectively treated drug-resistant gonorrhea and MRSA in both laboratory and mouse models, acting through previously unseen mechanisms such as destabilizing bacterial cell membranes and targeting membrane synthesis pathways. This innovation momentum is linked intrinsically to mounting global bacterial infections. According to the Global Burden of Disease Study 2019, 7.7 million people succumbed to bacterial infections in 2019.
Increased governmental funding and stringent regulatory frameworks promoting antibiotic stewardship are incentivizing pharmaceutical companies to channel investment toward adaptive drug delivery systems and formulation advancements, thus opening lucrative avenues.
The chief restriction impacting the antibiotics market growth stems from the labyrinthine interplay of stringent regulatory requirements and economic disincentives that together throttle antibiotic innovation and market entry. Amidst the skyrocketing prevalence of AMR and the surging urgency for effective medications, regulatory agencies worldwide have imposed increasingly rigorous clinical trial standards to ensure safety and efficacy, such as the FDA’s insistence on narrow non-inferiority margins below 10% for antibiotic approval.
Another example is that of India, where, in February 2025, the central government decided to classify all antimicrobial medicines, including antibiotics, as “new drugs” under the new regulatory framework. The move effectively removes state-level approval authority and centralizes manufacturing approvals with the Central Drugs Standard Control Organization (CDSCO), in response to the country’s severe antimicrobial resistance (AMR) crisis, which contributes to roughly 600,000 deaths annually.
These exacting standards and processes have led to the ultimate rejection of promising candidates such as iclaprim, which after years of costly phase 3 trials, was deemed insufficiently effective, illustrating how these regulatory hurdles delay or entirely halt innovative antibiotic development.
The antibiotics market participants can explore the meeting point of artificial intelligence (AI)-enabled drug discovery and collaborative licensing and partnership models, which collectively expedite the development and commercialization of precision antibiotics tailored for resistant bacterial strains.
Unlike conventional antibiotic development, hindered by long cycles and high costs, AI-driven platforms leverage machine learning algorithms to predict bacterial resistance mechanisms and optimize compound efficacy, significantly compressing timelines and boosting R&D productivity.
For instance, in September 2024, Phare Bio, in collaboration with MIT's Collins Lab and Harvard’s Wyss Institute, was awarded US$27s million by ARPA-H to accelerate its AI-powered antibiotic discovery platform, enhance clinical precision through millions of new training data points, and develop 15 novel preclinical AI-generated antibiotic candidates while creating the first open-source database for AI-based antibiotic research.
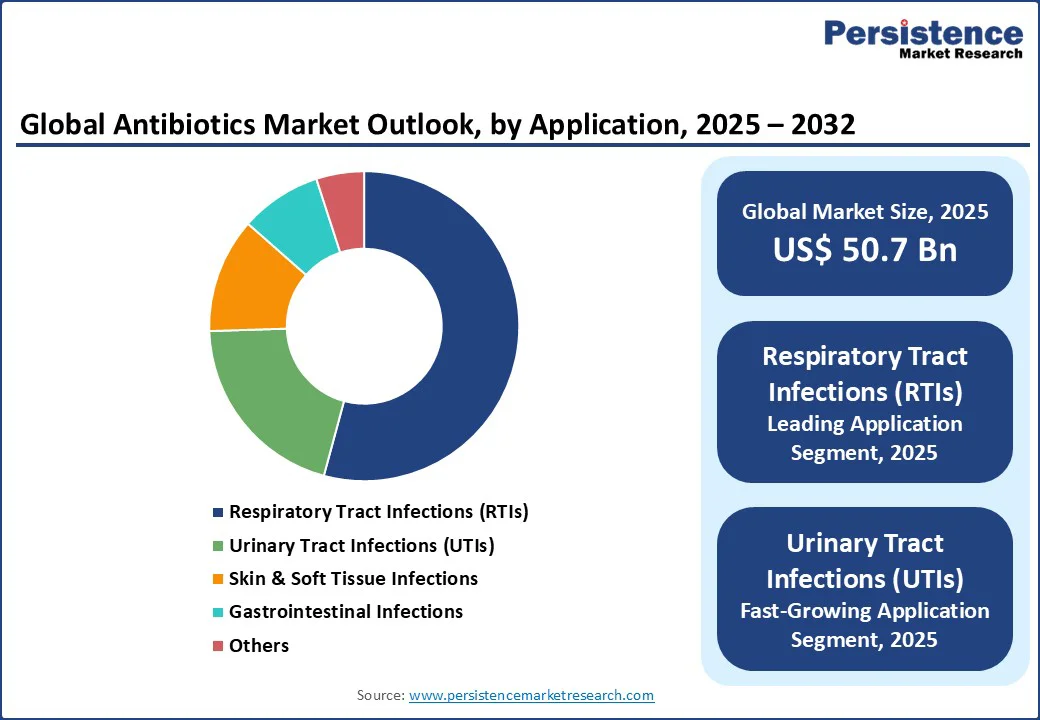
The respiratory infections segment is leading the application category with 54.2% market revenue share in 2025. This dominance is mainly the result of the widespread global prevalence of respiratory tract infections (RTIs) exacerbated by factors such as rising pollution levels, seasonal viral outbreaks, and the long-term impact of the COVID-19 pandemic, that has heightened susceptibility to secondary bacterial infections.
For example, data analysis of the Global Burden of Disease Study 2021 revealed that the global number of new cases of upper respiratory tract infections (URIs) was 12.8 billion in 2021. Innovations in inhaled antibiotics and rapid diagnostic tools have enhanced treatment efficacy and patient compliance, especially in chronic respiratory conditions such as cystic fibrosis and chronic obstructive pulmonary disorder (COPD), where bacterial colonization demands precision therapies.
In the drug class category, penicillin, with an estimated market revenue share of approximately 26.0%, is set to dominate. The top position of this segment is largely attributable to its established efficacy, broad-spectrum activity against gram-positive and gram-negative bacteria, and affordability, making it the frontline antibiotic for common bacterial infections such as streptococcal pharyngitis, pneumonia, and skin infections. The development of novel penicillin derivatives and advanced oral formulations further reiterates the growth potential of the penicillin segment.
For instance, researchers at the INEOS Oxford Institute discovered in May 2022 that derivatives of penicillin can inhibit the SARS-CoV-2 main protease, suggesting β-lactam antibiotics may be repurposed to target viral enzymes. The findings highlighted a novel approach to COVID-19 therapeutics by leveraging the acylating mechanism of penicillin derivatives to inactivate the viral protease. A beneficial trend is the increasing preference for semi-synthetic variants that combat evolving resistance patterns, which generates new opportunities for market players.
The evolving antibiotic resistance landscape has spiked the demand for newer, extended-spectrum cephalosporins, with leading pharmaceutical companies advancing innovative pipeline candidates such as Basilea Pharma’s Zevtera (ceftobiprole), which was approved by the FDA in 2024 for serious bacterial infections.
In the U.K., four cephalosporin antibiotics were reclassified as first-line treatments in February 2025, revising prescribing guidelines to better position these drugs in standard therapeutic protocols. This update reflects a strategic shift aimed at optimizing antibiotic use and ensuring more effective management of common infections. Apart from this, the movement towards combination therapies and innovations in parenteral drug delivery systems has further augmented the clinical versatility of cephalosporin, facilitating deeper market penetration.
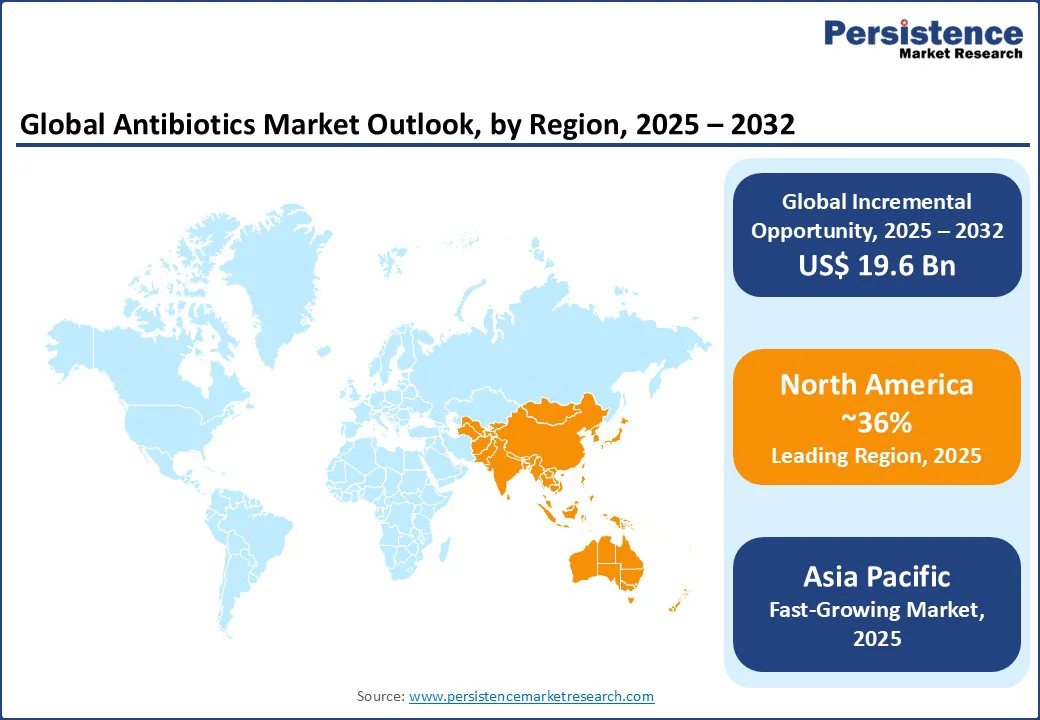
North America is likely to remain the largest regional market for antibiotics, forecast to hold a share of nearly 36.0% in 2025, due to a high prevalence of bacterial infectious diseases and a growing aging population requiring frequent and complex antibiotic treatments across the U.S. and Canada.
The U.S. leads the regional market growth on account of innovations facilitated by policies and acts such as the FDA's implementation of the Food and Drug Administration Safety and Innovation Act (FDASIA), titled Generating Antibiotic Incentives Now (GAIN), which extends exclusivity for infectious disease products, accelerating approvals for critical drugs such as ceftaroline and delafloxacin.
The robust pharmaceutical and biotech R&D ecosystem in the region has fostered the development of next-generation antibiotics targeting multidrug-resistant pathogens, supported by extensive surveillance networks that optimize stewardship protocols. High healthcare expenditure, insurance reimbursement policies favoring new antibiotics, and partnerships between biotech and healthcare providers boost market prospects further.
Asia Pacific is the fastest growing region, projected to outpace other regional markets with a CAGR surpassing 7.0% through 2032. This growth is fueled by a diverse population, high burden of infectious diseases, and increasing access to healthcare services amid rapid socioeconomic development in the region. India and China serve dual roles as the world’s largest antibiotic producers and the largest consumers, positioning the region as a global epicenter of generic antibiotics manufacturing and export.
Government initiatives promoting local production, coupled with improving insurance policies and expanding hospital infrastructure, have ballooned the demand for affordable yet effective antibiotics. Innovative public health strategies, including national AMR action plans integrated with digital surveillance and expanding community outreach, illustrate the proactive approach of governments in the region aimed at balancing increased access with resistance control.
Europe is anticipated to secure about one-fifth of the antibiotics market share in 2025, backed by mature healthcare systems, stringent regulatory oversight, and extensive antibiotic stewardship programs that encourage rational use of medications while also encouraging innovation. Germany, the U.K., and France dominate sales, propelled by robust public healthcare funding and an aggressive adoption of outcome-based procurement strategies rewarding clinical and stewardship benefits over competitive pricing.
The European Medicines Agency (EMA)’s streamlined approval pathways for unmet medical needs are designed to expedite market entry for novel antibiotics, including ceftobiprole and lefamulin, reinforcing the commitment of the EU to combating AMR while ensuring patient access. At the same time, clinical adoption of combination therapies and precision antibiotics is also gaining traction in the continent, supported by insurance structures that reimburse cutting-edge treatments.
Characterizing the global antibiotics market landscape is the convergence of technological innovation, strategic alliances, and evolving regulatory frameworks, which is creating a dynamic milieu that rewards both agility and scale. A critical driver is the surge in strategic collaborations and mergers and acquisitions (M&A), as these activities enable companies to pool R&D expertise, share regulatory risks, and expand geographic reach, accelerating the development and commercialization of novel antibiotics in the process.
Another key competitive trend is the integration of AI and machine learning to expedite antibiotic discovery and optimize clinical trial designs, addressing the urgent need for drugs effective against multi-drug-resistant pathogens. The adoption of breakthrough β-lactamase inhibitor combinations and innovative drug delivery systems has further enhanced therapeutic efficacy and patient compliance, marking a shift toward precision antibiotics.
The global antibiotics market is projected to reach US$ 50.7 billion in 2025.
The accelerated innovation in novel and broad-spectrum antibiotics and the mounting crisis of antimicrobial resistance (AMR) worldwide are driving the market.
The market is poised to witness a CAGR of 4.8% from 2025 to 2032.
The development of AI-driven platforms leverage machine learning algorithms to predict bacterial resistance mechanisms and optimize compound efficacy and increasing support for novel antibiotic drugs by regulatory bodies are key market opportunities.
Abbott Laboratories, AbbVie Inc., and Armata Pharmaceuticals Inc. are some key players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Drug Class
By Route of Administration
By Distribution Channel
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author