ID: PMRREP12154| 195 Pages | 22 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

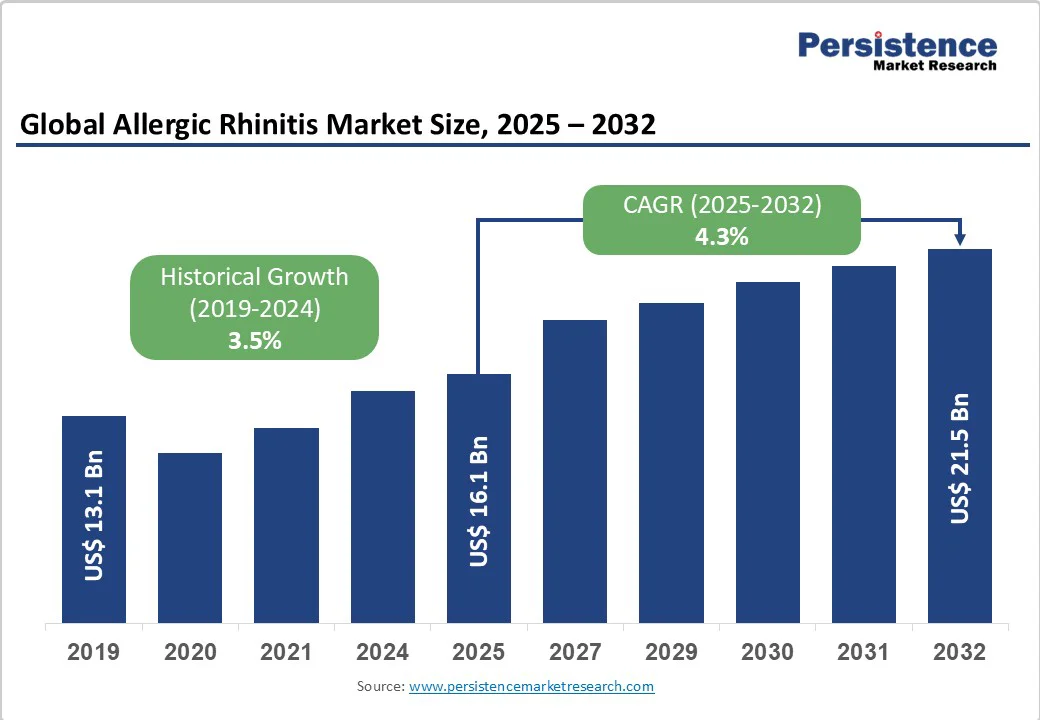
The global allergic rhinitis market size is likely to be valued at US$16.1 Bn in 2025 and is expected to reach US$21.5 Bn by 2032, growing at a CAGR of 4.3% during the forecast period from 2025 to 2032, driven by the prevalence of allergic respiratory conditions, rising urban pollution, and longer pollen seasons.
Nasal pharmacotherapies lead revenue generation, while immunotherapies and biologics drive high-value growth. Expanding telehealth, digital distribution, and OTC access further boost adoption and innovation-led market expansion.
| Key Insights | Details |
|---|---|
| Allergic Rhinitis Market Size (2025E) | US$16.1 Bn |
| Market Value Forecast (2032F) | US$21.5 Bn |
| Projected Growth (CAGR 2025 to 2032) | 4.3% |
| Historical Market Growth (CAGR 2019 to 2024) | 3.5% |
Allergic rhinitis affects 10-30% of the population in many countries, with urbanization, pollution, and climate change extending pollen seasons. This high prevalence increases demand for symptomatic treatments, particularly OTC and prescription nasal sprays, which account for more than 70% of total market revenue. Higher prevalence also drives physician visits and diagnosis rates, expanding the specialty therapy segment.
Allergy immunotherapy (subcutaneous and sublingual) and biologics targeting type 2 inflammation are expanding in indications and reimbursement coverage. Allergy immunotherapy is growing at an annual rate of approximately 8-9%, while biologics such as dupilumab continue to receive label expansions for upper airway diseases. These innovations increase per-patient revenue and broaden the addressable patient population.
OTC regulatory switches, expanding retail distribution, and online pharmacy growth improve patient access. Telehealth-enabled consultations shorten the path to diagnosis and prescription, particularly for immunotherapies and biologics. These trends reduce barriers to purchase, improve adherence, and contribute to overall market expansion.
Branded symptomatic treatments face margin compression due to generics and payer cost controls. Volume growth may continue, but pricing power is limited. Mid-single-digit annual growth in volume often coincides with margin pressure, especially in mature markets with high OTC penetration.
Immunotherapy and biologic programs involve high R&D costs and uncertain adoption. Regulatory approval delays or limited reimbursement could slow revenue recognition. Manufacturing complexity for allergen extracts also poses operational and quality risks.
Immunotherapy penetration remains low in APAC. Growing clinical infrastructure and increasing awareness provide opportunities to capture new patient populations. This expansion could add hundreds of millions in incremental revenue by 2032.
Biologics for type 2 inflammation and nasal polyposis offer premium pricing potential. Wider label indications, strong real-world evidence, and payer support in North America and Europe could generate several hundred million in revenue by 2030, particularly in well-reimbursed markets.
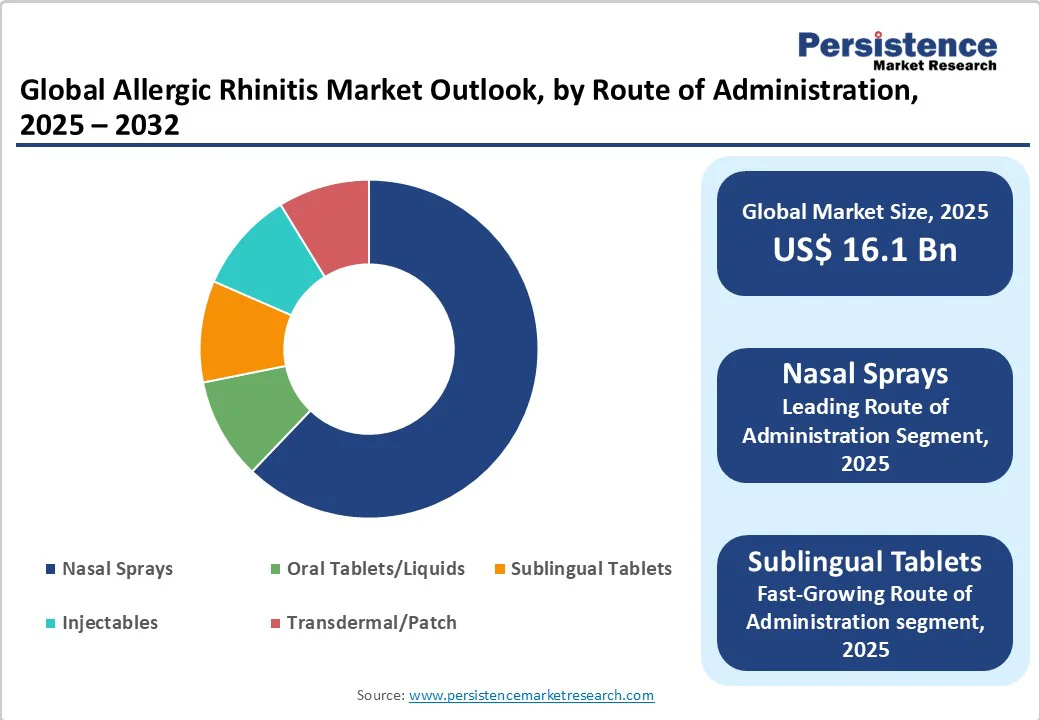
Nasal pharmacotherapies, including intranasal corticosteroids (fluticasone, mometasone) and nasal antihistamines (azelastine, olopatadine), dominate the allergic rhinitis market, accounting for over 69.6% of total market revenue in 2025.
Their wide OTC availability and strong prescription base ensure steady usage across both seasonal and perennial allergic rhinitis patients. Nasal sprays provide rapid symptomatic relief, are easy to administer, and have well-established safety profiles, strengthening patient adherence and repeat purchases.
For example, fluticasone nasal sprays by GSK and mometasone products by Sanofi are among the top-selling formulations in North America and Europe. This segment growth is reinforced by routine physician recommendations and public awareness campaigns, highlighting the benefits of nasal corticosteroids over oral medications in reducing nasal congestion, sneezing, and rhinorrhea.
Allergy immunotherapies, both subcutaneous (SCIT) and sublingual (SLIT), and biologics are the fastest-growing segment, expanding at an annual CAGR of 8-9%. Key drivers include increasing adoption of guideline-recommended allergen immunotherapy, availability of standardized allergen products, and biologic label expansions targeting severe phenotypes.
Biologics such as dupilumab (Regeneron/Sanofi) and omalizumab (Roche/Novartis) are increasingly prescribed for patients with comorbid conditions such as chronic rhinosinusitis with nasal polyps. SLIT tablets, including ALK-Abelló’s Grazax and Stalergenes’ Oralair, provide home-administered therapy options, improving convenience and patient adherence. Rising awareness of disease-modifying potential, rather than purely symptomatic relief, drives demand in mature and emerging markets alike.
Nasal sprays are the predominant route for allergic rhinitis treatment due to their efficacy, convenience, and rapid onset of action. This segment encompasses intranasal corticosteroids, antihistamines, and combination sprays, which together account for the majority of revenue in intranasal therapies. Nasal sprays remain a preferred option for both physicians and patients because they deliver medication directly to the site of inflammation.
For example, fluticasone propionate nasal spray and azelastine hydrochloride sprays are commonly prescribed for both seasonal and perennial rhinitis. OTC availability, such as over-the-counter fluticasone or mometasone sprays in the U.S. and U.K., ensures high-volume sales. Furthermore, nasal sprays are often recommended as first-line therapy in clinical guidelines, contributing to continued high uptake.
Sublingual tablets (SLIT) and injectable biologics are experiencing above-average growth due to convenience, efficacy, and expanding clinical indications. SLIT tablets, such as Grazax and Oralair, allow home administration without the need for clinic visits, which improves patient adherence and accessibility. Injectable biologics such as dupilumab and omalizumab are prescribed for severe allergic phenotypes, including patients with chronic rhinosinusitis with nasal polyps or severe uncontrolled rhinitis.
The segment growth is supported by label expansions, guideline endorsements, and improved reimbursement coverage in developed markets. Combined, these therapies represent a premium segment with higher per-patient revenue, attracting increased investment from pharmaceutical companies focused on specialty allergy treatments.
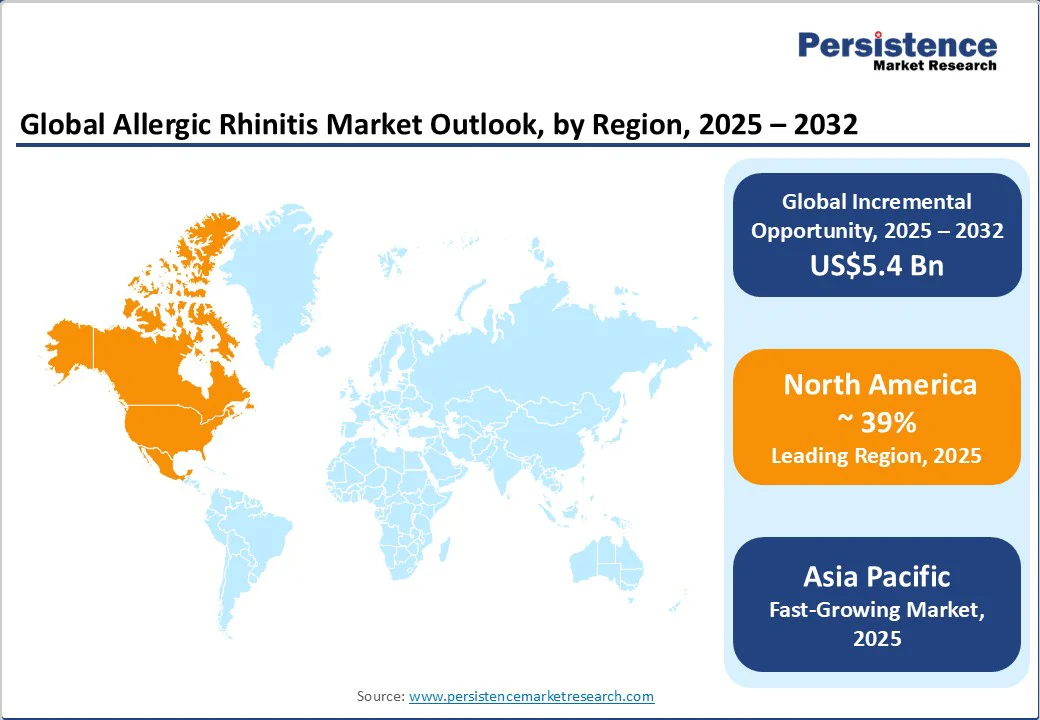
North America, led by the U.S., remains the largest market for allergic rhinitis, accounting for 39% of global revenue. High per-patient healthcare spending, robust insurance coverage, and widespread adoption of biologics and immunotherapies contribute to the region’s market dominance.
Nasal pharmacotherapies, including intranasal corticosteroids such as fluticasone (GSK) and mometasone (Sanofi), maintain strong OTC and prescription sales, while biologics such as dupilumab (Regeneron/Sanofi) and omalizumab (Roche) are increasingly prescribed for patients with severe allergic phenotypes.
Key growth drivers in North America include expanding immunotherapy adoption, ongoing biologic label expansions for adolescents and severe rhinitis cases, and wide OTC product accessibility. Over the past five years, the U.S. FDA has approved several sublingual immunotherapy (SLIT) tablets, including ALK-Abelló’s Oralair and Stalergenes’ Grazax, broadening treatment options for home administration. Telehealth platforms, integrated by major pharmacy chains and digital prescription services, further enhance patient access and treatment adherence.
Recent developments reinforce the region’s market growth. In 2024, Regeneron and Sanofi announced expanded dupilumab indications for chronic rhinosinusitis with nasal polyps, boosting specialty segment revenue. In 2023, ALK-Abelló launched new allergen SLIT tablets targeting pediatric and adolescent populations, expanding the immunotherapy market.
Retail pharmacies, including CVS Health and Walgreens, strengthened digital consultation services and integrated OTC allergy therapy programs in 2023-2024, improving patient engagement and convenience. Investments in North America focus on M&A, licensing agreements, and digital patient support services, with companies emphasizing educational programs to promote immunotherapy adoption and biologic adherence.
Europe is characterized by strong immunotherapy adoption, particularly in Germany, the U.K., France, and Spain, which collectively contribute the majority of regional revenue. Standardized allergen products dominate the market, while nasal pharmacotherapies continue to see widespread prescription use. Favorable reimbursement policies in countries such as Germany and France support immunotherapy uptake, and well-established allergy specialist networks facilitate diagnosis and access to advanced treatment options.
Regulatory harmonization under the European Medicines Agency (EMA) streamlines cross-border approvals, accelerating the commercialization of new allergen therapies. Distribution through retail and hospital pharmacy networks supports both OTC and prescription nasal sprays, while specialist clinics drive the adoption of SCIT and SLIT treatments.
Recent developments in Europe include Stallergenes Greer’s 2023 expansion of its immunotherapy portfolio in Germany with new SLIT formulations for grass pollen and dust mite allergies. DBV Technologies’ advanced Viaskin Peanut and other transdermal patch trials for allergy immunotherapy in France and the U.K., reflecting innovation in non-injectable delivery systems.
M&A activity in 2023-2024 included licensing agreements between ALK-Abelló and local European players to expand allergen coverage and standardize product offerings. Investments focus on licensing, commercialization, and clinical trials, with companies prioritizing unmet needs in pediatric populations and severe chronic allergic rhinitis.
Asia Pacific (APAC) is the fastest-growing regional market for allergic rhinitis, driven by rising diagnosis rates, urbanization, increased pollution, and growing awareness of allergic respiratory conditions. Key markets include China, Japan, India, and emerging ASEAN countries. Manufacturing advantages in China and India support cost-effective production of nasal pharmacotherapies and immunotherapy products, while expanding healthcare infrastructure improves patient access across urban and rural regions.
Regulatory frameworks vary across the region. Japan has well-established approval processes for immunotherapy, whereas China and India are expediting approvals for SLIT tablets and biologics, facilitating early market entry. Growing retail penetration, coupled with online pharmacy platforms and telehealth consultations, helps bridge access gaps, particularly in rural areas.
Recent developments highlight APAC’s growth trajectory. In 2024, ALK-Abelló launched SLIT products in China, marking the first large-scale rollout of standardized allergen tablets. GSK expanded OTC nasal spray distribution in India in 2023, increasing access to corticosteroid and antihistamine treatments.
Regeneron and Sanofi entered partnership agreements in 2023 to introduce dupilumab for severe allergic rhinitis in Japan, leveraging local distribution networks and insurance coverage. Investments in APAC are focused on scaling manufacturing, expanding clinical programs, and forming local partnerships, particularly to meet rising demand among the growing middle-class population for both symptomatic and disease-modifying therapies. With improving awareness, reimbursement, and regulatory support, APAC’s allergic rhinitis market is expected to maintain rapid growth in the coming years.
The global allergic rhinitis market is a mix of large pharmaceutical firms dominating symptomatic treatments and specialist immunotherapy companies controlling standardized allergens. Retail OTC channels are competitive and fragmented, while immunotherapy and biologics remain concentrated, supporting higher margins for specialists. Leading firms pursue product diversification, vertical specialization, and channel integration, combining R&D innovation with licensing deals to accelerate market access and manage manufacturing complexity.
The market size is estimated at US$16.1 Billion in 2025.
The allergic rhinitis market is projected to reach US$21.5 Billion by 2032, reflecting steady growth.
Key trends include rising adoption of immunotherapies and biologics, increasing OTC nasal spray use, expansion of sublingual immunotherapy tablets (SLIT) for home administration, growth in telehealth-based consultations, and regional expansion in Asia Pacific.
Nasal pharmacotherapies lead the market, contributing over 69.6% of revenue, with intranasal corticosteroids and antihistamines driving widespread OTC and prescription adoption.
The allergic rhinitis market is expected to grow at a CAGR of 4.3% between 2025 and 2032, reflecting steady demand for both symptomatic treatments and disease-modifying therapies.
Major players include ALK-Abelló A/S, GlaxoSmithKline (GSK), Sanofi, Regeneron Pharmaceuticals, and Stallergenes Greer.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Treatment Type
By Route of Administration
By Distribution
By Disease Type
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author