ID: PMRREP30413| 177 Pages | 8 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

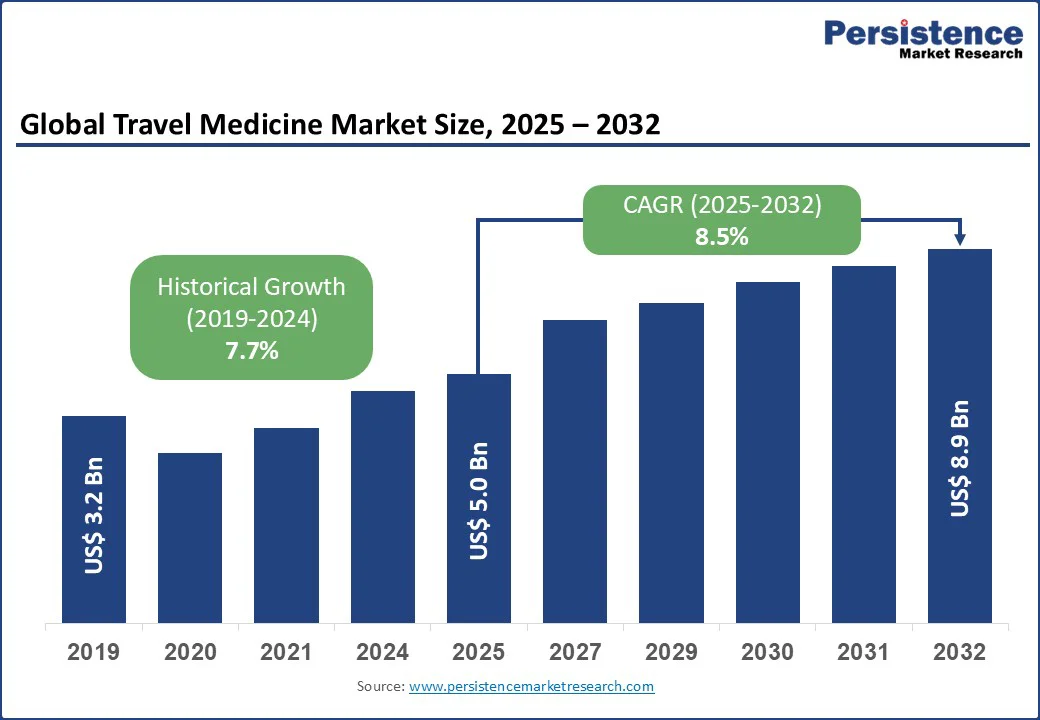
The global travel medicine market size is likely to be valued at US$5.0 Bn in 2025 and reach US$8.9 Bn by 2032, registering a CAGR of 8.5% during the forecast period from 2025 to 2032.
Key Industry Highlights:
| Global Market Attribute | Key Insights |
|---|---|
| Travel Medicine Market Size (2025E) | US$5.0 Bn |
| Market Value Forecast (2032F) | US$8.9 Bn |
| Projected Growth (CAGR 2025 to 2032) | 8.5% |
| Historical Market Growth (CAGR 2019 to 2024) | 7.7% |
The travel medicine industry is witnessing strong growth fuelled by rising global tourism, heightened awareness of travel-related health risks, and growing demand for preventive measures. Increasing uptake of vaccines, travel insurance, and specialized advisory services supports market expansion, while government initiatives enhance accessibility and safety for international travellers.
The global surge in awareness of travel-related infectious diseases is a primary driver of the travel medicine market. According to the World Health Organization, approximately 1.8 billion international tourist arrivals were recorded in 2024, with significant risks of vector-borne diseases such as malaria and dengue in tropical destinations.
The rising prevalence of these conditions, particularly among adventure and business travelers, drives demand for travel-specific vaccines and preventive measures. In North America, a significant share of international travelers seek pre-travel consultations, necessitating advanced vaccine development and distribution technologies to meet demand.
Technological advancements in vaccine formulation and delivery are propelling market growth. Modern systems, such as Valneva’s single-dose cholera vaccine, offer improved efficacy and convenience, enhancing consumer satisfaction.
Clinical studies have shown that travel vaccines significantly reduce the risk of severe illness compared to unvaccinated individuals, boosting consumer confidence. The integration of digital health platforms and telemedicine for travel consultations supports adoption in preventive care and advisory services.
Government health initiatives and increased funding for global health programs also drive market expansion. In India, national schemes such as Ayushman Bharat have expanded access to preventive healthcare, increasing demand for travel vaccines. In North America, favorable regulatory policies, such as the CDC’s approval of travel health guidelines, incentivize manufacturers to invest in high-efficacy vaccines, further fueling market growth.
High costs of travel-specific vaccines and consultations continue to hinder widespread adoption, particularly in cost-sensitive markets such as the Asia Pacific. Vaccines for diseases such as yellow fever and typhoid, often required for travel to endemic regions, can be expensive per dose, posing a barrier for budget travelers.
The high cost of research and development for specialized vaccines, such as those certified by the FDA, adds to production expenses, with advanced manufacturing systems requiring significant upfront investment. Additionally, ongoing costs for quality testing and compliance with international health regulations, such as WHO’s prequalification standards, increase the total cost of ownership. For healthcare providers in resource-limited regions, such as parts of Latin America and Southeast Asia, these financial burdens limit scalability, restricting access to premium travel medicine services.
The need for skilled healthcare professionals to administer vaccines and provide travel health advice also poses a challenge. Delivering high-quality travel medicine requires expertise in infectious diseases and traveler risk assessment, and industry surveys have reported a shortage of trained specialists in Asia Pacific healthcare facilities. This skills gap, combined with high training costs, restricts the adoption of advanced services in developing regions, slowing market growth.
The development of innovative and accessible travel vaccines presents significant growth opportunities, particularly by enabling effective prevention for health-conscious travelers. Single-dose and combination vaccines, such as those developed by Sanofi and Valneva, offer convenient alternatives to multi-dose regimens, reducing reliance on complex schedules.
These innovations address accessibility concerns and appeal to the growing health-conscious demographic, particularly in North America and Europe. For instance, Dynavax Technologies’ hepatitis B vaccine is gaining traction in travel clinics, exemplifying the shift toward efficient solutions.
The rise of digital health platforms for travel medicine consultations offers another growth avenue. Telemedicine services, rich in real-time risk assessment tools, are increasingly used to provide pre-travel advice and vaccination recommendations.
Market studies highlight strong growth in telehealth adoption for travel medicine, driving demand for integrated health solutions. Companies such as Pfizer are capitalizing on this trend by offering digital vaccination record systems for travelers.
The growing adoption of blockchain for vaccination record transparency also enhances market potential. Companies such as Abbott Laboratories are integrating IoT-based traceability systems, ensuring regulatory compliance and traveler trust. This trend supports market expansion by addressing consumer concerns about vaccine authenticity and improving healthcare efficiency.
The global travel medicine market is segmented into routine vaccinations, travel-specific vaccines, booster shots, and vaccination records. Travel-specific vaccines dominate, holding approximately 42% of the travel medicine market share in 2025, due to their targeted protection against diseases such as yellow fever and typhoid. These vaccines are widely used for their high efficacy, making them a critical choice for travelers to endemic regions.
Booster shots are the fastest-growing segment, driven by increasing demand for long-term immunity in frequent travelers. Their role in maintaining protection, particularly in North America and Europe, boosts adoption in high-value markets.
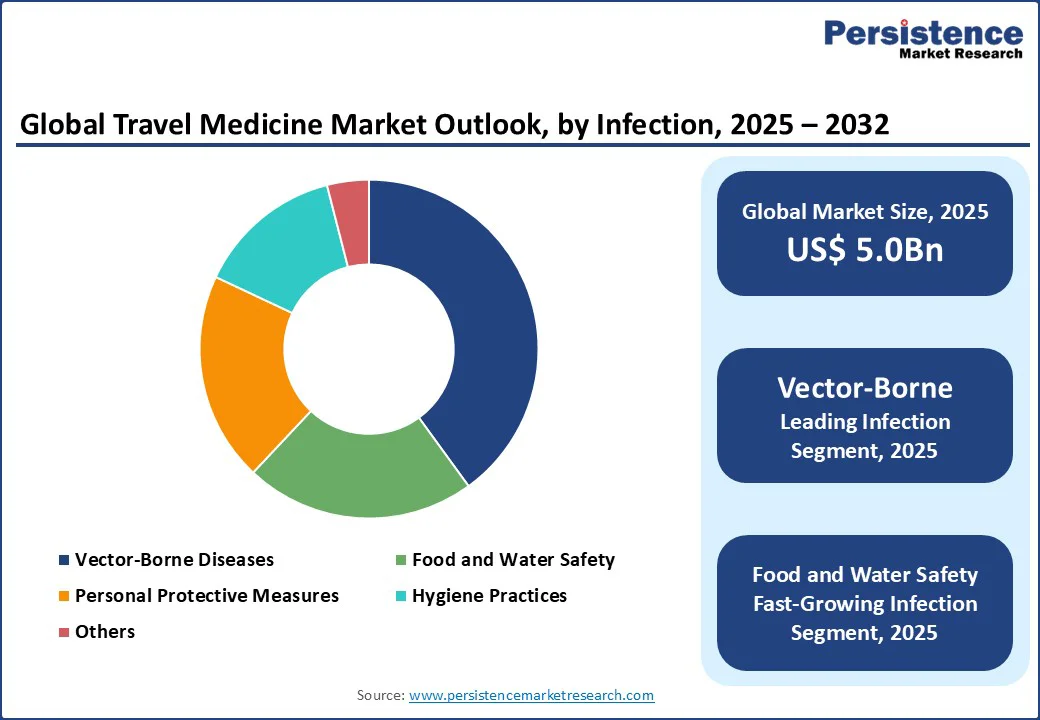
By infection type, the travel medicine market is divided into vector-borne diseases, food and water safety, personal protective measures, and hygiene practices. Vector-borne diseases lead with a 40% share in 2025, driven by their high prevalence in travel health strategies for diseases such as malaria and dengue. Preventive measures are critical for traveler safety, with the majority of global travel medicine efforts focused on this category.
Food and Water Safety is the fastest-growing segment, fueled by rising awareness of diseases such as traveler’s diarrhea and hepatitis A. The increasing emphasis on safe consumption practices, particularly in the Asia Pacific and Europe, drives demand for specialized preventive solutions.
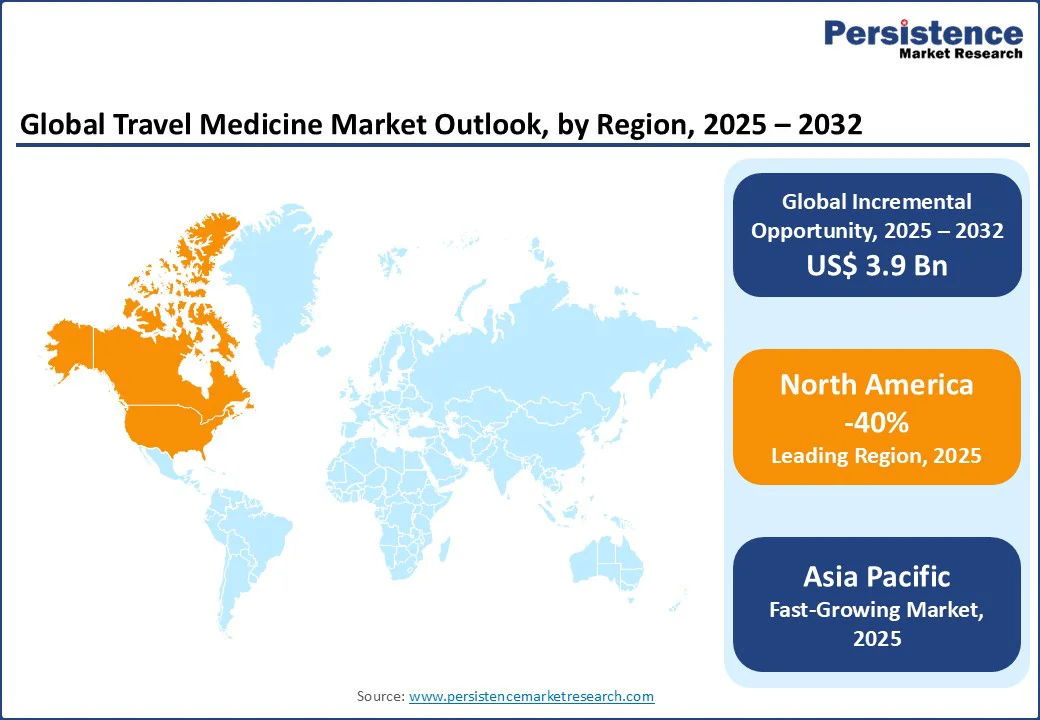
In North America, the U.S. holds a dominant position with approximately 40% market share in the travel medicine market, supported by high consumer awareness of travel-related health risks and a well-established healthcare infrastructure.
Demand for travel-specific vaccines continues to expand, largely driven by the steady rise in international tourism and the growing emphasis on preventive healthcare solutions. Leading pharmaceutical players, including Pfizer and Merck & Co., remain central to this growth by consistently introducing innovative, high-efficacy vaccines tailored to the needs of diverse traveler demographics.
Consumer preferences are also evolving, with increasing attention on digital health and transparent vaccine traceability. Companies such as Abbott Laboratories are leveraging this trend by integrating CDC-certified protocols and digital health record systems to enhance trust and compliance.
The emphasis on health consciousness, alongside stringent FDA regulations, reinforces the adoption of high-quality and safety-certified vaccines. Moreover, favorable regulatory policies surrounding travel health claims provide strong incentives for manufacturers to further invest in advanced vaccine technologies, strengthening the region’s leadership in the global travel medicine market.
Europe is a key region in the global travel medicine market, led by Germany, France, and the U.K., driven by regulatory support and high consumption of travel health services. Germany holds a large share within Europe, supported by strong sales from leading brands such as GSK and Sanofi. The EU’s strict regulations on vaccine safety, such as the European Medicines Agency’s guidelines, foster innovation and compliance, encouraging the adoption of certified vaccines and digital health platforms across major markets.
In the U.K., market growth is driven by the rising preference for travel-specific vaccines in adventure tourism. Products such as Valneva’s cholera vaccine are gaining popularity for their convenience and efficacy. Meanwhile, France is witnessing increased demand for digital consultation services, with companies such as Novartis offering specialized solutions. Regulatory support for preventive health practices across Europe further enhances market prospects.
Asia Pacific is emerging as the fastest-growing region in the travel medicine market, with China, India, and Japan playing pivotal roles. In India, rising health awareness and supportive government healthcare initiatives, including Ayushman Bharat, are boosting demand for affordable and accessible travel vaccines.
Companies such as Takeda Pharmaceutical are at the forefront, supplying cost-effective yet high-efficacy vaccines to meet this demand. In China, the rapid expansion of both domestic and international tourism is driving the travel medicine market, with leading brands such as Dynavax Technologies strengthening their presence in travel clinics by offering high-quality vaccine solutions.
Japan, meanwhile, is developing a specialized market for premium-grade vaccines catering to frequent business travelers, where AstraZeneca is becoming increasingly prominent. Alongside these trends, rising healthcare investments, coupled with the integration of advanced digital health platforms, continue to accelerate regional adoption and market growth.
The global travel medicine market is highly competitive, with global and regional players competing on innovation, efficacy, and accessibility. The rise of single-dose vaccines and digital health platforms intensifies competition, as companies aim to meet stringent regulatory and consumer standards. Strategic partnerships, regulatory certifications, and investments in vaccine technologies are key differentiators.
The travel medicine market is projected to reach US$5.0 Bn in 2025.
Rising health awareness, international travel expansion, and government health initiatives are the key market drivers.
The Travel Medicine market is poised to witness a CAGR of 8.5% from 2025 to 2032.
Innovation in vaccine development and digital health platforms are the key market opportunities.
Pfizer, GSK, and Sanofi are among the key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
| Customization and Pricing |
|
By Vaccination Type
By Infection
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author