ID: PMRREP34825| 285 Pages | 11 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

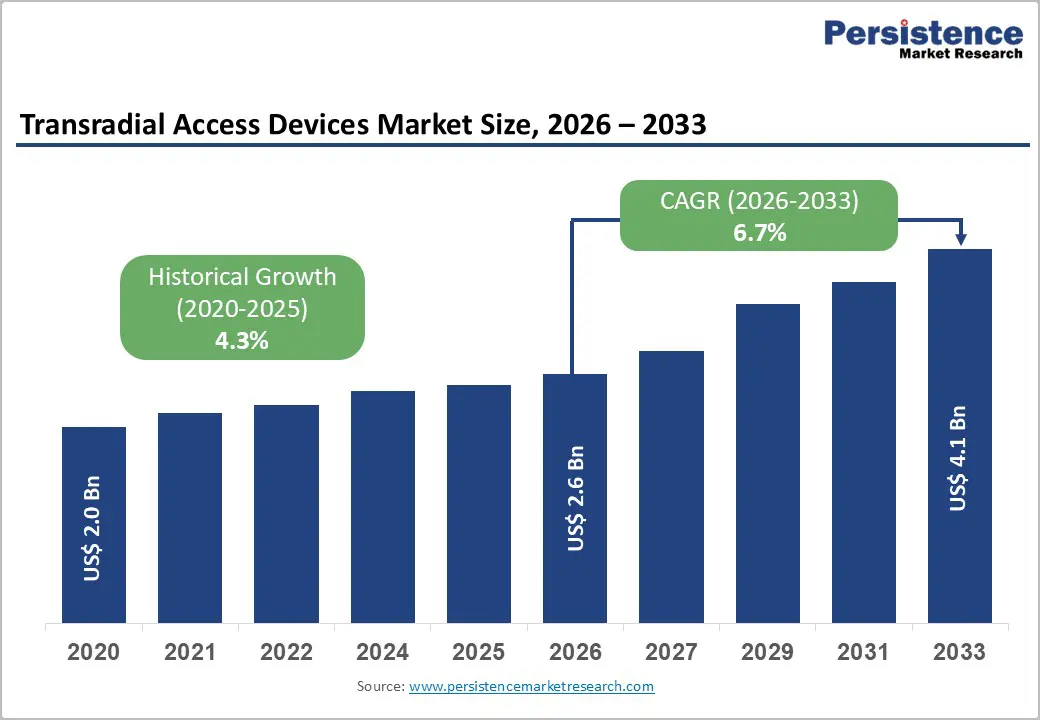
The global transradial access devices market size is expected to be valued at US$ 2.6 billion in 2026 and projected to reach US$ 4.1 billion by 2033, growing at a CAGR of 6.7% between 2026 and 2033.
The market is expanding steadily, supported by rising adoption in interventional cardiology and radiology. These devices enable minimally invasive entry through the radial artery, allowing clinicians to perform procedures such as angioplasty, stent placement, diagnostic angiography, and peripheral vascular interventions. Their role in modern cardiovascular care continues to strengthen as hospitals and catheterization laboratories increasingly favor safer, patient-friendly access routes over traditional femoral approaches.
The growing preference for minimally invasive techniques is due to reduced bleeding risk, quicker recovery, and shorter hospital stays. Continuous technological improvements are producing more precise, ergonomic, and reliable devices, improving outcomes and workflow efficiency. Adoption is also expanding into neurology and peripheral interventions, while training programs and education initiatives are boosting physician confidence and accelerating broader clinical uptake.
| Key Insights | Details |
|---|---|
| Transradial Access Devices Market Size (2026E) | US$ 2.6 billion |
| Market Value Forecast (2033F) | US$ 4.1 billion |
| Projected Growth CAGR (2026 - 2033) | 6.7% |
| Historical Market Growth (2020 - 2025) | 4.3% |
One of the primary drivers of market expansion is the growing preference for minimally invasive procedures among healthcare providers and patients. Transradial access offers significant advantages, including reduced bleeding risk, shorter recovery times, and greater patient comfort compared with traditional femoral access. As awareness of these benefits grows, more interventional cardiologists and radiologists are adopting transradial techniques, thereby increasing demand for specialized access devices. This trend reflects a broader shift in the healthcare landscape towards safer and more efficient surgical practices.
Technological advancements in medical devices are significantly propelling the growth of the transradial access devices market demand. Innovations in device design, such as improved catheter materials and enhanced delivery systems, are making procedures safer and more efficient. These advancements facilitate more accessible access to the vascular system and minimize complications associated with traditional methods. As manufacturers continue to invest in research and development, the introduction of next-generation transradial access devices is expected to attract more healthcare professionals, further driving market expansion and improving patient outcomes.
The rising incidence of cardiovascular diseases globally is another critical driver of growth in the transradial access devices market. As the prevalence of conditions such as coronary artery disease and peripheral artery disease increases, the demand for effective interventional procedures rises correspondingly.
Transradial access has emerged as a preferred approach for many of these interventions due to its safety profile and patient benefits. Consequently, healthcare systems are increasingly adopting transradial techniques to address the growing burden of cardiovascular diseases, fueling the market for transradial access devices and related technologies.
One significant factor impeding growth in the transradial access devices market is the need for greater awareness and training among healthcare professionals. Despite the advantages of transradial access, many interventional cardiologists and radiologists may still prefer traditional femoral access due to familiarity and established protocols. This reluctance may stem from a need for more comprehensive training programs that emphasize the benefits and techniques of transradial procedures. As a result, the slow adoption of these devices in clinical practice can hinder market growth, as healthcare providers may need to fully utilize the potential of transradial access.
Another factor that challenges the market growth is the potential for complications and technical difficulties associated with the procedure. While transradial access is generally considered safe, issues such as radial artery spasm, hematoma formation, and access site complications can occur, leading to adverse patient outcomes. The technical skill required to perform transradial procedures effectively may deter some practitioners from adopting this approach. These complications can engender hesitancy among healthcare providers, thereby limiting the widespread adoption and use of transradial access devices across various interventional settings.
One significant future opportunity for growth in the transradial access devices market lies in the expansion into new clinical applications beyond traditional cardiology. As healthcare providers increasingly recognize the versatility of transradial access techniques, there is potential for their adoption in various fields, including neurology and peripheral vascular interventions.
Manufacturers can tap into new revenue streams by developing specialized devices tailored for these applications and addressing the growing demand for minimally invasive procedures across diverse medical disciplines. This expansion can enhance the overall market landscape and encourage broader clinical adoption of transradial techniques.
Integrating advanced technologies into the transradial access devices market presents a promising opportunity for market growth. Innovations such as robotics, artificial intelligence, and real-time imaging can enhance the precision and effectiveness of transradial procedures.
By incorporating these technologies, manufacturers can develop devices that improve procedural outcomes, reduce complications, and streamline workflows for healthcare professionals. Combining transradial access with telemedicine solutions could facilitate remote consultations and monitoring, further expand the potential applications of these devices and attract a more comprehensive range of healthcare providers to adopt transradial techniques.
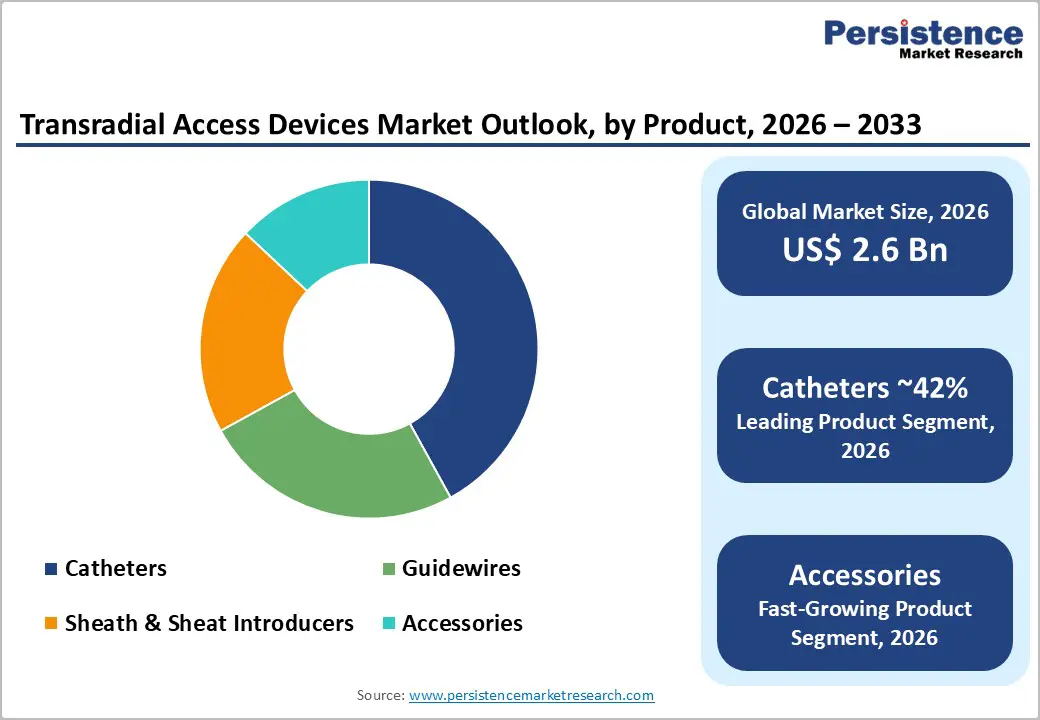
Catheters are projected to hold the largest share of the transradial access devices market, reaching nearly 42% by 2026, supported by the growing global burden of cardiovascular diseases and accelerating preference for minimally invasive interventions. Transradial catheterization is increasingly favored for coronary angiography and angioplasty because it lowers bleeding complications, improves patient comfort, and enables faster ambulation compared with femoral approaches. Continuous innovation in catheter tip geometry, shaft flexibility, hydrophilic coatings, and torque response has further strengthened adoption by improving navigation through complex vascular anatomy and enhancing procedural success rates. Manufacturers are also focusing on radial-specific curve designs that provide better coronary engagement while minimizing arterial trauma.
A notable industry development occurred in July 2023 when ALVIMEDICA received CE Mark approval for its Alvision Kaplan radial portfolio, featuring stability-focused curves intended to optimize operator control and patient safety. Such product launches, combined with increasing physician training in radial techniques, continue to reinforce catheter leadership within the overall market.
Hospitals are expected to dominate the end-use landscape of the transradial access devices market, capturing about 40.5% of the market by 2026. This leadership reflects the high volume of cardiovascular procedures performed in hospital catheterization laboratories and the rising incidence of coronary artery disease worldwide. Hospitals increasingly adopt radial-first strategies due to their clinical advantages, including reduced vascular complications, shorter inpatient stays, faster post-procedural recovery, and improved patient satisfaction scores. Large multispecialty hospitals are also better positioned to invest in advanced imaging suites, hybrid operating rooms, and staff training programs that support widespread adoption of transradial procedures. Favorable reimbursement structures for inpatient and outpatient cardiac interventions further strengthen this segment’s growth outlook. Policy developments have reinforced momentum; for instance, reimbursement initiatives introduced in late 2022 encouraged hospital-based procedural billing and streamlined coverage for interventional care. Together, infrastructure readiness, payment support, and the expansion of cardiac caseloads ensure that hospitals remain the primary purchasers and users of transradial access technologies.
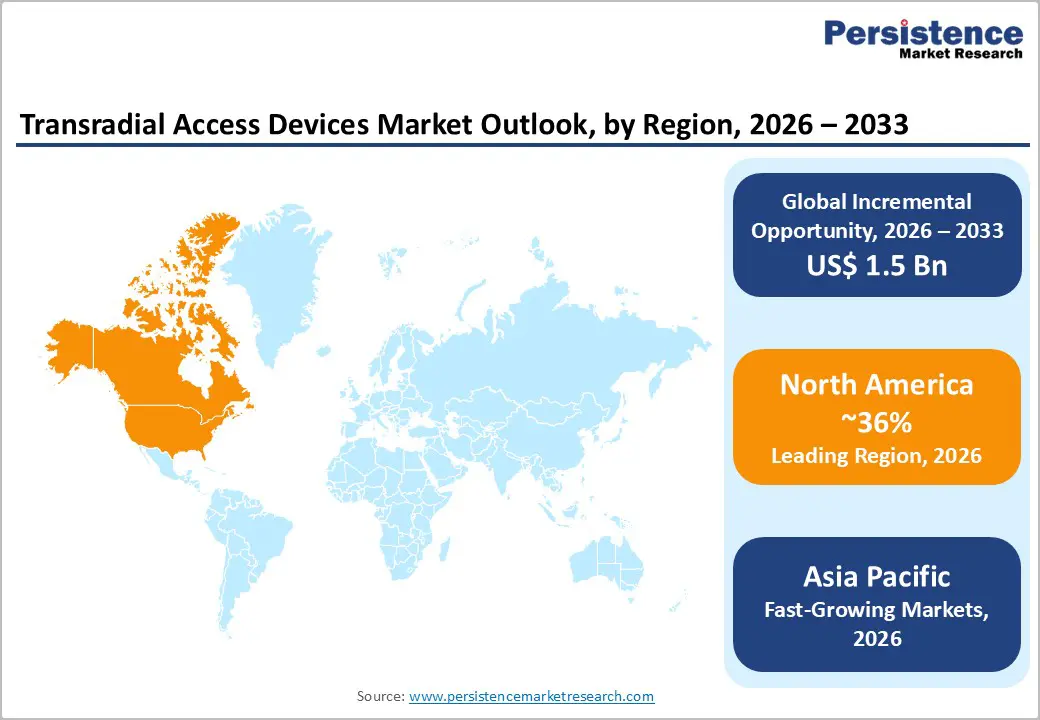
North America continues to represent a mature and high-value market for transradial access devices, supported by strong adoption of minimally invasive cardiovascular procedures and well-established catheterization laboratory infrastructure. The United States leads regional demand, driven by a high prevalence of coronary artery disease, large procedural volumes for angiography and percutaneous coronary intervention, and broad physician familiarity with radial-first protocols. Academic medical centers and integrated delivery networks frequently adopt new catheter designs, hydrophilic sheaths, and imaging-guided access systems that improve procedural efficiency and patient comfort. Favorable reimbursement frameworks for outpatient cardiac interventions and short-stay procedures further reinforce uptake. Continuous professional training programs, society-led guidelines, and clinical trial activity encourage consistent use of radial approaches over femoral access.
Canada also contributes steadily through public-sector investments in hospital modernization and cardiovascular care capacity. Together, clinical preference shifts, supportive payment policies, and technology-driven workflow improvements sustain stable expansion across the region.
Europe demonstrates strong penetration of transradial techniques, supported by long-standing clinical preference for radial access in coronary procedures and widespread guideline endorsement from cardiology societies. Countries such as France, Germany, Italy, and the United Kingdom remain key contributors owing to high procedural volumes and extensive public health care networks. European hospitals emphasize patient safety, early mobilization, and cost-effective care pathways, factors that favor radial approaches over femoral alternatives. Procurement programs within national health systems increasingly prioritize devices that reduce complication rates and shorten length of stay.
Regional manufacturers and multinational suppliers actively introduce catheter platforms, low-profile sheaths, and access kits tailored for complex anatomies and elderly populations. Training workshops and fellowship programs across tertiary cardiac centers further accelerate physician proficiency. Eastern European markets are gradually expanding as catheterization laboratory capacity grows and public investment in cardiovascular services increases, supporting a broader base for sustained regional demand.
Asia-Pacific is emerging as one of the fastest-growing regions for transradial access devices, driven by increasing incidence of cardiovascular disease, rising interventional procedure volumes, and rapid hospital infrastructure development. China, Japan, India, and South Korea account for a substantial share of regional demand, supported by government investments in cardiac care networks and modernization of catheterization laboratories. Large urban hospitals increasingly adopt radial-first strategies to manage high patient throughput while limiting post-procedure complications and inpatient bed utilization. Local manufacturers are strengthening their presence by offering cost-competitive catheters and sheath systems, thereby improving accessibility across public and private hospitals. Training initiatives led by professional societies and teaching hospitals are broadening physician expertise in radial techniques, especially in secondary cities. The growth of medical tourism hubs in Southeast Asia also contributes to increased demand, as international patients seek minimally invasive cardiac interventions. These combined structural and clinical factors underpin strong growth momentum throughout the region.
The competitive landscape of the transradial access market is characterized by several key players striving to innovate and capture market share. For instance, Terumo Corporation launched the Glidecath® 6F Radial Access Catheter in 2022, enhancing the ease of access and maneuverability during procedures. These innovations reflect the ongoing efforts of companies to develop advanced, user-friendly devices that cater to the growing demand for minimally invasive procedures, thereby driving competition within the market.
The global transradial access devices market is projected to be valued at US$ 2.6 Bn in 2026.
Rising minimally invasive procedures, lower bleeding risks, faster recovery, expanding cardiac interventions, technological improvements, and increasing physician training programs worldwide.
The global market is expected to witness a CAGR of 6.7% between 2026 and 2033.
Expanding use in neurology and peripheral interventions, emerging-market hospital investments, innovative device designs, radial-first clinical protocols, and large-scale physician education initiatives.
North America is the leading region in the global transradial access devices market.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn and Volume (if Available) |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By End Use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author