ID: PMRREP35352| 187 Pages | 23 May 2025 | Format: PDF, Excel, PPT* | Healthcare

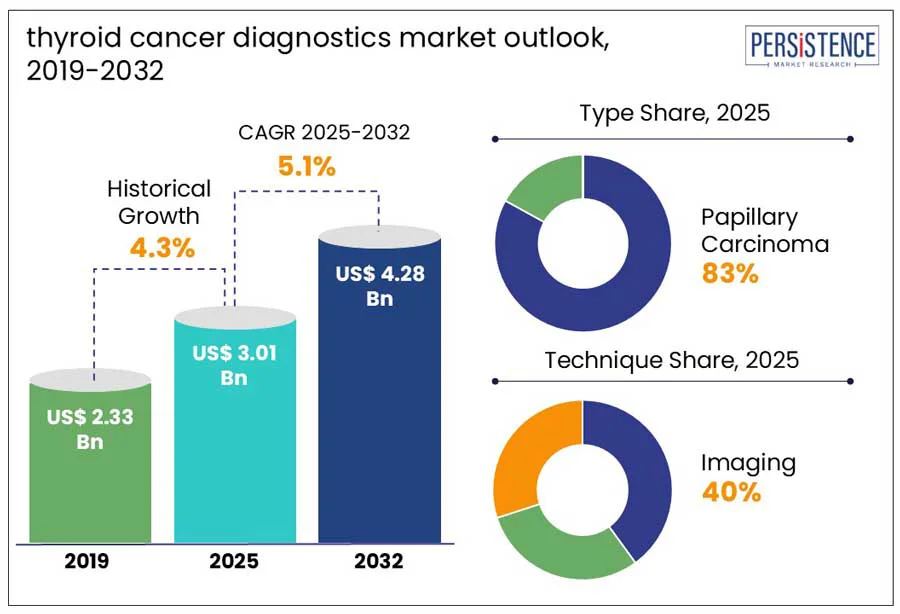
The global thyroid cancer diagnostics market size is projected to rise from US$ 3.01 Bn in 2025 to US$ 4.28 Bn by 2032. The market is further anticipated to register a CAGR of 5.1% during the forecast period from 2025 to 2032. According to the Persistence Market Research report, market growth is driven by the rising incidence of thyroid-related disorders, autoimmune illnesses such as Grave’s disease, and thyroid cancer; growing awareness of preventive healthcare and increase in screening and routine health checkups; aging population; advancements in diagnostic technologies; and higher healthcare spending fueled by rising disposable incomes.
The American Cancer Society identifies several key tools for diagnosing thyroid cancer, including blood tests (thyroid hormone and TSH levels), imaging techniques (ultrasound, CT, MRI, and PET scans), laryngoscopy, Fine Needle Aspiration (FNA) biopsy, and radioiodine scans used to detect thyroid tissue and monitor cancer spread. FNA, especially when guided by ultrasound, provides accurate tissue sampling while reducing the need for invasive procedures. Additionally, next-generation sequencing (NGS) services have become valuable in identifying genetic mutations linked to thyroid cancers, paving the way for personalized treatment approaches, thereby driving growth in the thyroid cancer diagnostics market.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Thyroid Cancer Diagnostics Market Size (2025E) |
US$ 3.01 Bn |
|
Market Value Forecast (2032F) |
US$ 4.28 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
5.10% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.30% |
The thyroid cancer diagnostics market is witnessing strong growth, driven by the increasing prevalence of thyroid cancer cases. A study published in August 2023 on ScienceDirect reports that thyroid cancer is the fifth most commonly diagnosed cancer globally and is nearly three times more prevalent in women than in men. According to the American Cancer Society's January 2025 report, approximately 44,020 new thyroid cancer cases are expected in the U.S. (12,670 in men and 31,350 in women) with an estimated 2,290 related deaths (1,090 men and 1,200 women). This rising incidence of thyroid cancer is a key driver in the growing demand for diagnostic tests and services.
This heightened focus on global health programs and awareness campaigns has significantly boosted demand for diagnostic services, as more individuals seek testing. January is observed as Thyroid Awareness Month, marked by various initiatives and events focused on promoting early detection and treatment of thyroid disorders. A May 2025 paper published by the National Library of Medicine (NLM) emphasized that combining at-home sample collection with telehealth services represents an innovative and scalable model. This approach enhances access to thyroid disorder screening and monitoring across diverse age groups and geographic regions, making early detection more convenient and widespread. Telehealth platforms such as Teladoc and Amwell have improved remote access to thyroid care.
The high cost of molecular and genetic testing remains a significant barrier to the widespread use of advanced thyroid cancer diagnostics, particularly in low- and middle-income countries. NGS tests, used to detect mutations in genes such as BRAF, RET, and RAS that cause thyroid cancer, are very expensive. A study by NLM in October 2024 found that in Nova Scotia, current tests for indeterminate thyroid nodules are 64% effective and cost US$ 6,431. Routine molecular testing improves accuracy to 89% but raises the cost to US$ 8,414, with an added cost of US$ 7,876 per surgery avoided. These tests will be more affordable if prices drop or insurance coverage expands.
In countries including the U.S., molecular panels, such as ThyroSeq v3 and Afirma GSC, are used but are often limited to specialized centers or academic hospitals, restricting access for rural patients. The high costs are driven by the use of advanced equipment such as Illumina sequencers, the need for skilled personnel, and the time-consuming process of data analysis. In countries including India and Brazil, molecular diagnostics are rarely reimbursed, and even in wealthier nations, insurance coverage is often limited. These challenges hinder the adoption of advanced diagnostics, leaving many patients reliant on less accurate methods such as FNAC and ultrasound, thereby increasing the risk of misdiagnosis and overtreatment.
Emerging diagnostic technologies, such as AI-based tools and non-invasive procedures, are set to drive the market. AI is increasingly being integrated into diagnostic workflows, offering faster, more accurate interpretations of imaging and pathology data. These tools can streamline diagnosis, reduce human errors, and lower reliance on conventional, often more expensive, diagnostic procedures such as FNA biopsies or extensive imaging. Liquid biopsy techniques also enable accurate and early detection of thyroid cancer markers in blood, offering a less invasive and more efficient alternative to tissue biopsies.
FNA biopsies for thyroid nodules are being replaced by molecular diagnostics, such as Afirma and ThyroSeq, alongside AI-assisted ultrasound analysis, thereby enhancing diagnostic accuracy and avoiding unnecessary surgeries. A May 2024 study by the NLM on AI-assisted diagnosis of early thyroid cancer markers supports this shift, confirming that AI plays a crucial role in improving the precision and efficiency of thyroid cancer diagnosis. By integrating ML, deep learning, and computer vision into conventional techniques, such as ultrasound, FNA, and molecular testing, AI enables early detection and management of thyroid cancer, thereby propelling the thyroid cancer diagnostics market.
The papillary carcinoma segment dominates and is expected to hold around 83% of the market share in 2025. It has the highest cure rate among all thyroid cancers, with a five-year survival rate of nearly 98%. Advances in ultrasound imaging, FNA biopsy, and molecular testing have improved early detection and treatment planning. The adoption of non-invasive and cost-effective diagnostics, such as FNA and genetic testing, is expanding in developing regions, thereby supporting the continued dominance of papillary carcinoma in the market.
Follicular carcinoma, though less common than papillary, is projected to be the fastest-growing segment in the market due to its higher malignancy potential, early metastasis to lungs and bones, and need for advanced diagnostics such as genetic testing and imaging. It typically affects individuals over 50 and requires complex treatment, including surgery, radioactive iodine, and TSH suppression. Rising awareness, improved diagnostic technologies, and a growing emphasis on early detection are driving segment growth over the forecast period.
The imaging segment is expected to dominate the thyroid cancer diagnostics market in 2025, accounting for around 40% of total revenue. Imaging techniques, such as ultrasound, CT, MRI, and PET scans, plays a crucial role in detecting, evaluating, and monitoring thyroid nodules and tumors. Ultrasound is extensively used to detect structural abnormalities in the thyroid, while other modalities, such as radioiodine scans, chest X-rays, and bone scans, help in detecting metastases. The integration of AI and high-resolution imaging has further enhanced diagnostic precision and efficiency. Despite emerging molecular tests, imaging remains the basis of thyroid cancer diagnostics due to its accessibility and precision. In April 2024, GE HealthCare expanded its strategic collaboration with Elekta by integrating MIM Software into Elekta’s radiation therapy systems to enhance imaging, treatment planning, and global oncology care delivery.
The biopsy segment is the fastest-growing in the market owing to its role in confirming cancer at the cellular level. Biopsy samples are increasingly utilized for NGS and biomarker analysis, enhancing diagnostic accuracy and supporting personalized care. The demand for minimally invasive, reliable, and definitive diagnostic methods continues to propel the adoption of biopsy techniques in thyroid cancer diagnostics.
North America is expected to lead the market with a revenue share of 43%, driven by its high prevalence, a well-developed healthcare infrastructure, and widespread use of molecular diagnostics. The region benefits from strong investments in medical research, supportive reimbursement frameworks, and proactive government initiatives for early detection. Companies such as Lifelabs, Dynacare, BioMark Diagnostics, and Theralase Technologies offer advanced technologies including imaging, genetic testing, and next-generation sequencing. North America is also a hub for innovative treatments, with drugs (Lenvatinib and Cabozantinib) and immunotherapies (Nivolumab and Pembrolizumab) already approved for thyroid cancer care. Continuous awareness efforts and access to cutting-edge diagnostic tools reinforce the region’s dominant position in the global market.
The U.S. dominates the market due to its large patient base, high healthcare spending, favorable insurance coverage, strong research infrastructure, and rapid adoption of advanced technologies such as genetic testing and AI-driven imaging.
Asia Pacific is expected to witness the fastest growth in the market over the forecast period. This surge is driven by a rising incidence of thyroid cancer, increased government healthcare spending, and rapid advancements in diagnostic technologies. Additionally, governments across Asia Pacific are actively promoting awareness campaigns to encourage early detection and timely treatment. BGI Genomics, Mindray Medical International, Samsung Medison, and Fujifilm Holdings Corporation are key players in the region.
Japan is anticipated to lead in Asia Pacific in 2025, driven by an aging population, heightened public awareness, increased investments in research, and robust government-supported cancer screening programs. In March 2018, Canon Medical Systems Corporation (formerly Toshiba Medical) launched the Aplio i900 ultrasound system, equipped with i18LX5 high-frequency transducer for advanced thyroid nodule evaluation.
Europe is anticipated to dominate and witness substantial growth in the market from 2025 to 2032. The market is projected to grow steadily, driven by increasing thyroid cancer cases, favorable healthcare policies, and the adoption of advanced diagnostic technologies. Substantial research funding, cross-border collaborations, and harmonized regulatory frameworks support innovation and the faster rollout of new diagnostic tools, thereby enhancing early detection and driving sustained market expansion across the region.
Germany is expected to lead in Europe thyroid cancer diagnostics market in terms of market share and revenue and will maintain its dominance during the forecast period. This is due to the rising incidence of thyroid nodules & cancer.
The global thyroid cancer diagnostics market is highly competitive, with global and domestic players offering a wide range of products and competing for a higher market share. Companies are investing in R&D and adopting growth strategies such as product innovations, strategic partnerships, and acquisitions. Major players include Thermo Fisher Scientific, Siemens Healthcare, GE HealthCare, and Roche.
The global market is projected to be valued at US$ 3.01 Bn in 2025.
The rising incidence of thyroid-related disorders, growing awareness of preventive healthcare, an increase in screening and routine health checkups, an aging population; advancements in diagnostic technologies, and higher healthcare spending fueled by rising disposable incomes.
The market is poised to witness a CAGR of 5.10% from 2025 to 2032.
AI is increasingly being integrated into diagnostic workflows, offering faster, more accurate interpretations of imaging and pathology data.
Major players in the thyroid cancer diagnostics industry include F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific, Inc., Siemens Healthcare GmbH, Bio-Rad Laboratories, Inc., and GE HealthCare.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Type
By Technique
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author