New Born Screening Market Size and Forecast Analysis
Market Overview
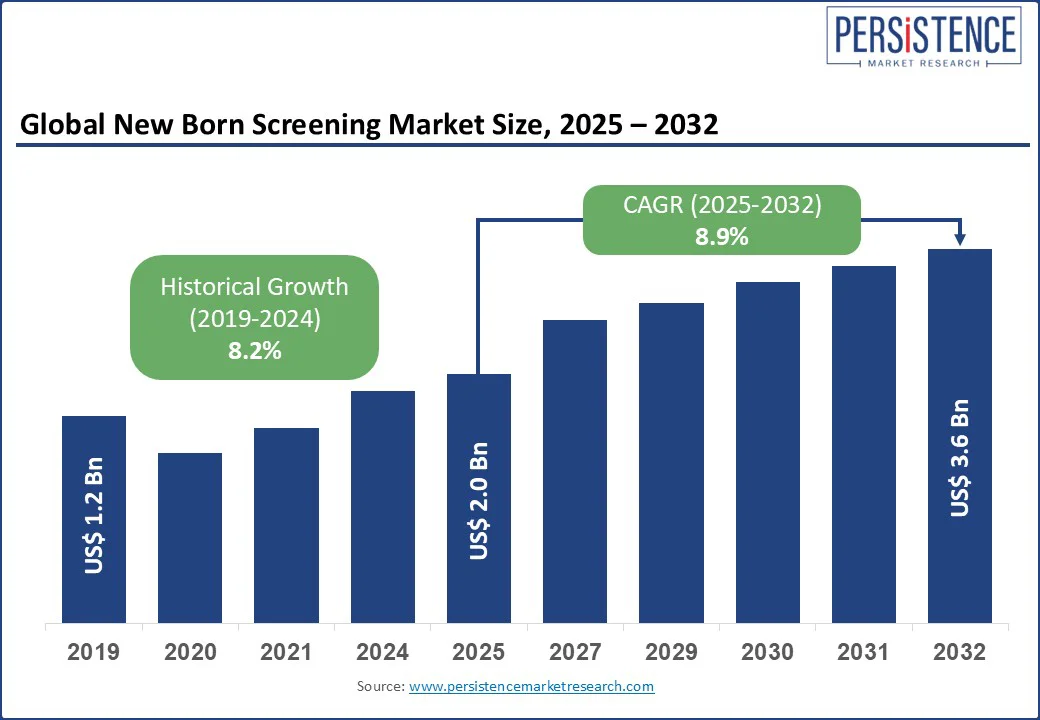
The global newborn screening market is likely to be valued at US$2.0 billion in 2025 to US$3.6 billion by 2032, registering a compound annual growth rate (CAGR) of 8.9% during the forecast period (2025–2032). This growth is driven by increasing awareness of early detection of genetic disorders in infants, advancements in neonatal diagnostics, and expanding government initiatives for mandatory newborn screening programs.
The newborn screening market is a critical segment of the pediatric healthcare industry, focused on congenital anomaly detection for infant health, targeting genetic, metabolic, and congenital disorders in newborns to enable timely intervention and treatment. North America holds the largest market share, driven by robust pediatric healthcare infrastructure and regulatory mandates, while the Asia Pacific is emerging as a high-growth region due to rising investments in neonatal testing and population growth.
Market Dynamics
Drivers
- Rising Incidence of Congenital Disorders Drives Newborn Screening Market: The escalating prevalence of congenital disorders significantly fuels the newborn screening market, as early detection of genetic disorders in infants is vital for preventing long-term disabilities. The World Health Organization reports that approximately 7.9 million infants are born each year with congenital disorders, including conditions such as newborn screening for cystic fibrosis, congenital hypothyroidism, and sickle cell disease, which can lead to severe health issues if not addressed early. This high incidence highlights the urgent need for comprehensive infant screening programs to identify disorders promptly, enabling timely interventions that enhance infant health outcomes. For instance, the CDC notes that U.S. newborn screening programs detect around 12,900 infants annually with conditions such as phenylketonuria (PKU), underscoring the importance of congenital anomaly detection. Advancements such as tandem mass spectrometry improve neonatal diagnostics precision, propelling market growth. As awareness of congenital disorders increases, the demand for accessible, effective newborn screening continues to surge, ensuring healthier outcomes for infants globally.
- Government Mandates Fuel Newborn Screening Market Growth: Mandatory newborn screening programs and public health initiatives significantly drive the newborn screening market by promoting early detection of genetic disorders in infants. Over 70 countries, including the U.S., Canada, and European nations such as the UK and Germany, enforce mandatory newborn screening programs to identify congenital disorders early, preventing long-term health issues.
For instance, the U.S. Health Resources and Services Administration (HRSA) recommends infant screening for over 30 disorders, including newborn screening for cystic fibrosis and severe combined immunodeficiency (SCID), ensuring standardized neonatal testing across states. According to the CDC, nearly 4 million U.S. newborns are screened annually, boosting market adoption. In Canada, programs such as Ontario’s Newborn Screening, which tests for 29 conditions, as noted by the Canadian Pediatric Society, enhance early intervention efforts for infant health.
The UK’s NHS Newborn Blood Spot Screening Programme, focusing on genetic screening for nine rare disorders, exemplifies how public health initiatives increase accessibility and awareness. Advanced neonatal diagnostics, such as next-generation sequencing, further improve diagnostic precision, supporting market growth.
Restraint
- High Costs of Advanced Technologies: The cost of instruments such as tandem mass spectrometers, often exceeding US$500,000, and recurring expenses for reagents pose financial barriers, particularly in low-income regions, limiting newborn screening and congenital anomaly detection in these areas.
Opportunities
- Development of Cost-Effective Technologies: Innovations in portable and low-cost neonatal diagnostics, such as point-of-care pulse oximeters, could increase accessibility for infant screening in resource-constrained settings, enhancing congenital anomaly detection. Innovations in portable screening solutions, such as Mylab’s MyNeoShield device launched in September 2023, enable rapid and cost-effective screening in remote areas.
- Integration of Artificial Intelligence (AI) and Machine Learning: AI-driven analytics can enhance diagnostic accuracy and streamline workflows. For instance, in 2023, Agilent Technologies partnered with AI firms to develop predictive models for metabolic disorder detection, reducing false positives by 15% in clinical trials. This technology is expected to drive market growth by improving efficiency and reliability in screening processes.
Category-wise Analysis
Product Insights
- Instruments dominate the newborn screening market, which has a 76% market share which is driven by the adoption of advanced equipment such as tandem mass spectrometers and next-generation sequencing platforms in developed markets, supporting neonatal diagnostics. These instruments enable high-throughput screening, critical for detecting multiple disorders efficiently. The high cost of instruments, coupled with recurring maintenance and calibration needs, contributes to their significant revenue share.
- Reagents is the fastest-growing segment. Their recurring demand for tests such as dry blood spot and enzyme-based assays ensures steady market contribution to infant health. Advances in reagent formulations, such as those by Bio-Rad Laboratories, improve test sensitivity, further driving adoption.
Technology Insights
- Tandem mass spectrometry leads the technology segment, capturing 25% of the newborn screening market share in 2024 due to its ability to screen multiple congenital disorders simultaneously with high accuracy. Widely used in developed markets such as the U.S. and Germany, this technology benefits from continuous improvements by companies such as Waters Corporation, enhancing its diagnostic capabilities and market dominance.
- Pulse oximetry is the fastest-growing technology. Its non-invasive nature and ability to detect critical congenital heart defects (CCHD) drive its adoption, particularly in regions mandating CCHD screening, such as the U.S. and U.K. Innovations by Masimo, including portable and cost-effective devices, are expanding its use in low-resource settings, contributing to rapid growth.
Test Type Insights
- Dry blood spot tests hold the largest share, at 46% in 2024, due to their widespread use in newborn screening for metabolic congenital disorders such as phenylketonuria (PKU) and hypothyroidism. Its simplicity, requiring only a few drops of blood collected on filter paper, makes it cost-effective and scalable, especially in large-scale programs.
- CCHD screening is the fastest-growing test type, driven by mandatory newborn screening programs in over 40 countries and the rising prevalence of congenital heart defects affecting infant health. Pulse oximetry-based CCHD tests, which are non-invasive and quick, are increasingly integrated into newborn screening protocols.
Regional Insights
North America Newborn Screening Market Trends
North America accounted for 38% of the global newborn screening market in 2024, with the U.S. leading due to its advanced pediatric healthcare system and mandatory newborn screening programs. The U.S. screens over 4 million newborns annually, covering 35 congenital disorders, as per HRSA guidelines. Key drivers include:
- Regulatory Support: The Newborn Screening Saves Lives Act ensures federal funding for infant screening programs, with US$20 million allocated in 2024.
- Technological Adoption: The U.S. leads in adopting tandem mass spectrometry and next-generation sequencing for neonatal diagnostics, with over 80% of screening labs equipped with advanced instruments.
- High Awareness: Public health campaigns have achieved a 95% screening compliance rate in the U.S., per CDC data, enhancing congenital anomaly detection.
Europe Newborn Screening Market Trends
Europe holds a 30% market share, with Germany, the UK, and France as leading countries for newborn screening.
- Germany: The country medical infrastructure screening for 19 congenital disorders, supported by the Federal Ministry of Health’s funding of €50 million annually. Adoption of pulse oximetry for CCHD screening drives neonatal testing growth.
- UK: The National Health Service (NHS) mandates infant screening for nine disorders, with a 98% compliance rate. Investments in DNA assay technologies for genetic screening are driving growth.
- France: France’s focus on rare disease detection, with €30 million invested in genetic screening in 2024, supports newborn screening market expansion.
Asia Pacific Newborn Screening Market Trends
- China: With over 17 million annual births, China’s investment in pediatric healthcare infrastructure, including US$1 Bn for mandatory newborn screening programs, drives market growth.
- India: The country’s National Health Mission aims to screen 10 million newborns annually by 2030, supported by partnerships with private players such as Trivitron Healthcare for congenital anomaly detection.
- Japan: Advanced technology adoption, including tandem mass spectrometry for neonatal diagnostics, and government subsidies for infant screening programs contribute to growth.
Competitive Landscape
The global newborn screening market is highly competitive. Companies such as Bio-Rad and Waters Corporation are investing in R&D to develop cost-effective reagents and portable instruments for neonatal testing. Trivitron Healthcare collaborates with regional governments in Asia to expand newborn screening access. GE Lifesciences and Masimo are focusing on emerging markets, establishing distribution networks in India and China for infant health solutions. These companies dominate due to their extensive product portfolios and global presence in pediatric healthcare and neonatal diagnostics.
Key Developments
- Agilent Technologies (2024): Launched a new tandem mass spectrometry system with enhanced sensitivity for congenital disorders.
- PerkinElmer Inc. (2023): Partnered with the Indian government to expand dry blood spot infant screening in rural areas.
- Natus Medical Inc. (2024): Introduced a portable pulse oximeter for CCHD screening, targeting low-resource settings for congenital anomaly detection.
Companies Covered in New-Born Screening Market
- Agilent Technologies Inc.
- AB SCIEX
- Natus Medical Inc.
- Covidien plc
- Trivitron Healthcare
- GE Lifesciences
- Masimo
- Waters Corporation
- PerkinElmer Inc.
- Bio-Rad Laboratories Inc.
Frequently Asked Questions
The new born screening market is projected to reach US$2.0 billion in 2025.
Rising incidence of congenital disorders and mandatory newborn screening programs, such as the U.S. RUSP, are key drivers.
The new born screening market is poised to witness a CAGR of 8.9% from 2025 to 2032.
Development of cost-effective neonatal diagnostics and expansion in emerging markets such as India and China are key opportunities.
Agilent Technologies Inc., AB SCIEX, Natus Medical Inc., Covidien plc, Trivitron Healthcare, GE Lifesciences, Masimo, Waters Corporation, PerkinElmer Inc., and Bio-Rad Laboratories Inc.
Global New Born Screening Market Report Scope
|
Report Attribute
|
Details
|
|
Historical Data/Actuals
|
2019-2024
|
|
Forecast Period
|
2025-2032
|
|
Units
|
Value: US$ Bn/Mn
|
|
2025 (E)
|
US$ 2.0 bn
|
|
2032 (F)
|
US$ 3.6 bn
|
|
Historical CAGR (2019-2024)
|
8.2%
|
|
Projected CAGR (2025-2032)
|
8.9%
|
|
Geographical Coverage
|
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East and Africa
|
|
Segmental Coverage
|
- Product Type
- Technology
- Test Type
- Region
|
|
Competitive Analysis
|
- Agilent Technologies Inc.
- AB SCIEX
- Natus Medical Inc.
- Covidien plc
- Trivitron Healthcare
- GE Lifesciences
- Masimo
- Waters Corporation
- PerkinElmer Inc.
- Bio-Rad Laboratories Inc.
- Others.
|
|
Report Highlights
|
- Market Forecast and Trends
- Competitive Intelligence and Share Analysis
- Growth Factors and Challenges
- Strategic Growth Initiatives
- Pricing Analysis
- Future Opportunities and Revenue Pockets
- Market Analysis Tools
|
|
Customization and Pricing
|
Available upon request
|
Market Segmentation
By Product Type
By Technology
- Tandem Mass Spectrometry
- Pulse Oximetry
- Enzyme-based assay
- DNA Assay
- Electrophoresis
- Other
By Test Type
- Dry Blood Spot Test
- CCHD
- Hearing Screen
By Region
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East and Africa

