ID: PMRREP33828| 207 Pages | 23 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

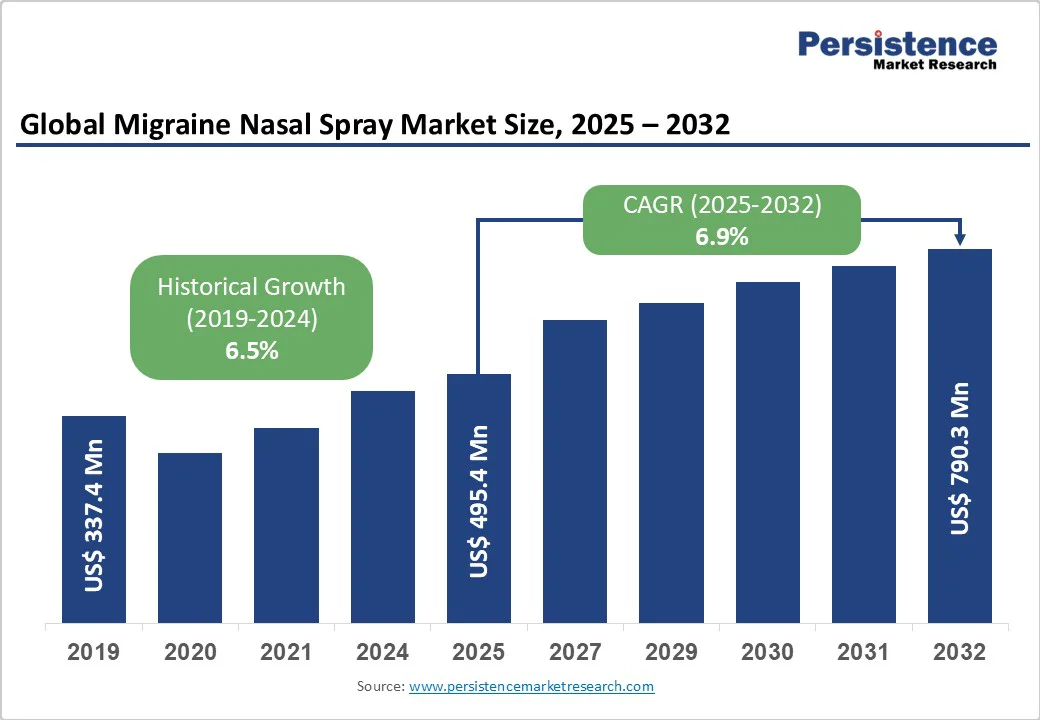
The global migraine nasal spray market size is likely to be valued US$ 495.4 Mn in 2025, growing to US$ 790.3 Mn by 2032 at a CAGR of 6.9% during the forecast period from 2025 to 2032.
The market is experiencing robust growth driven by the increasing prevalence of migraines, rising demand for fast-acting and non-invasive treatment options, and advancements in nasal spray formulations. The need for rapid relief from debilitating migraine symptoms, particularly in acute care settings, has significantly boosted the adoption of nasal sprays across various demographics.
| Key Insights | Details |
|---|---|
| Migraine Nasal Spray Market Size (2025E) | US$ 495.4 Mn |
| Market Value Forecast (2032F) | US$ 790.3 Mn |
| Projected Growth (CAGR 2025 to 2032) | 6.9% |
| Historical Market Growth (CAGR 2019 to 2024) | 6.5% |
The increasing prevalence of migraines globally is a primary driver of the Migraine Nasal Spray Market. According to the World Health Organization, migraines affect approximately 1 in 7 people worldwide, with a higher prevalence among women (18%) than men (6%). This widespread condition creates a substantial demand for effective and fast-acting treatments.
Nasal sprays offer a rapid onset of action, typically within 15-30 minutes, compared to oral medications, which can take up to an hour, making them ideal for patients seeking immediate relief from acute migraine symptoms. This is particularly critical for individuals experiencing nausea or vomiting, which can hinder the effectiveness of oral drugs.
The growing adoption of triptans, such as sumatriptan and zolmitriptan, in nasal spray formulations has further accelerated market growth. For instance, sumatriptan nasal sprays, such as those marketed by companies such as Merck, have gained significant traction due to their proven efficacy in clinical trials, with over 60% of patients reporting pain relief within two hours.
Additionally, the rise in healthcare awareness and the increasing availability of over-the-counter migraine treatments in retail pharmacies have boosted consumer access, further driving market expansion. The surge in demand for non-invasive delivery systems, coupled with innovations in preservative-free formulations, continues to propel the market forward, particularly in developed regions with advanced healthcare systems.
The high costs associated with the development and regulatory approval of migraine nasal sprays pose a significant restraint on market growth. Developing nasal spray formulations requires advanced pharmaceutical technologies, rigorous clinical trials, and specialized delivery systems to ensure bioavailability and safety.
These processes involve substantial financial investment, often exceeding millions of dollars, which can be a barrier for smaller companies and startups entering the market. Additionally, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose stringent requirements for drug approval, including extensive safety and efficacy testing.
Compliance with these standards, along with the need for specialized manufacturing facilities, increases overall costs and extends development timelines.
For instance, the approval process for new triptan-based nasal sprays can take several years, with costs escalating due to multiple phases of clinical trials and post-market surveillance requirements. Smaller firms often struggle to meet these financial demands, limiting their ability to compete with established players such as Thermo Fisher Scientific and Merck.
Furthermore, the complexity of integrating advanced delivery mechanisms, such as metered-dose systems, adds to production costs, which can deter innovation and slow market expansion, particularly in cost-sensitive regions.
Advancements in preservative-free and combination formulations present significant growth opportunities for the Migraine Nasal Spray Market. Preservative-free nasal sprays reduce the risk of nasal irritation and allergic reactions, making them a preferred choice for patients with sensitive nasal passages.
These formulations align with growing consumer demand for safer and more tolerable treatment options. Additionally, combination formulations that integrate triptans with other active ingredients, such as alpha-adrenergic agonists, offer enhanced efficacy by targeting multiple migraine pathways, providing faster and more comprehensive symptom relief.
For example, companies such as Abcam and Cayman Chemical are investing in R&D to develop combination nasal sprays that combine triptans with anti-inflammatory agents, improving patient outcomes. These innovations reduce development timelines and costs by leveraging modular formulation designs that can be adapted for various patient needs.
Furthermore, the integration of smart delivery systems, such as AI-optimized dosing mechanisms, is enhancing the precision and efficiency of nasal sprays, improving patient compliance. As the demand for personalized and patient-centric treatments grows, these advancements are expected to drive market expansion, particularly in regions with high healthcare expenditure and innovation focus, such as North America and Europe.
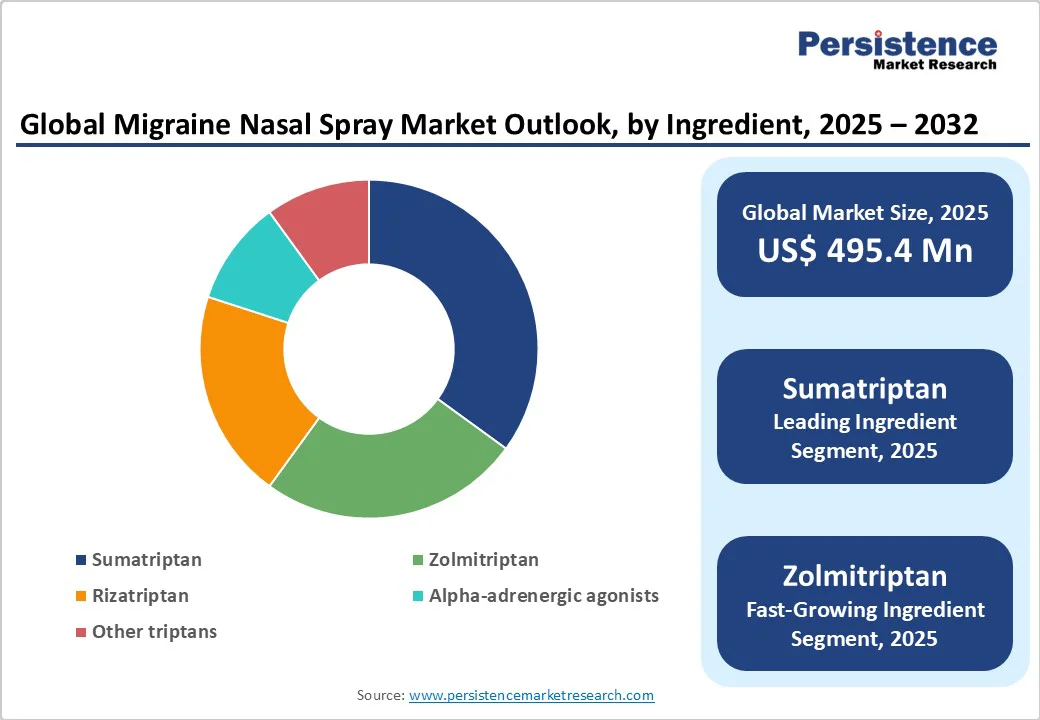
Sumatriptan dominates and is expected to account for approximately 48% share in 2025. Its dominance is driven by its established efficacy, widespread clinical use, and availability in both branded and generic forms.
Sumatriptan nasal sprays, such as those offered by Merck, are highly effective in treating acute migraines, with clinical studies reporting pain relief in over 60% of patients within two hours. Its ability to provide rapid relief and its compatibility with nasal delivery systems make it a preferred choice for both patients and healthcare providers.
Zolmitriptan is the fastest-growing segment, driven by its favorable pharmacokinetic profile and increasing adoption in preservative-free formulations. Zolmitriptan nasal sprays offer a slightly faster onset of action compared to sumatriptan in some studies, making them appealing for patients seeking rapid relief.
The growing focus on personalized medicine and the development of zolmitriptan-based combination therapies are further accelerating its adoption, particularly in North America and Europe.
Single-agent formulations lead the market, holding approximately 45% of the share in 2025. Their dominance stems from their simplicity, cost-effectiveness, and targeted therapeutic action, making them suitable for a wide range of patients. Single-agent nasal sprays, such as those containing sumatriptan, are widely used in retail pharmacies due to their ease of use and established clinical efficacy.
Preservative-free formulations are the fastest-growing segment, driven by increasing consumer demand for safer and more tolerable treatment options. These formulations minimize the risk of nasal irritation, making them ideal for patients with chronic migraines or sensitivities. Companies such as Thermo Fisher Scientific are investing in preservative-free technologies, which are gaining traction in developed markets with stringent safety standards.
Retail pharmacies dominate the market, contributing nearly 50% of revenue in 2025. Their widespread accessibility, coupled with the growing availability of over-the-counter migraine nasal sprays, drives their dominance. Retail pharmacies offer convenience and immediate access, making them the preferred choice for patients seeking relief from acute migraine.
Online pharmacies are the fastest-growing segment, fueled by the rise of e-commerce and increasing consumer preference for digital healthcare solutions. The convenience of home delivery, coupled with competitive pricing and access to a wide range of products, is driving rapid adoption. The Asia Pacific region, with its growing digital infrastructure, is a key contributor to this segment’s growth.
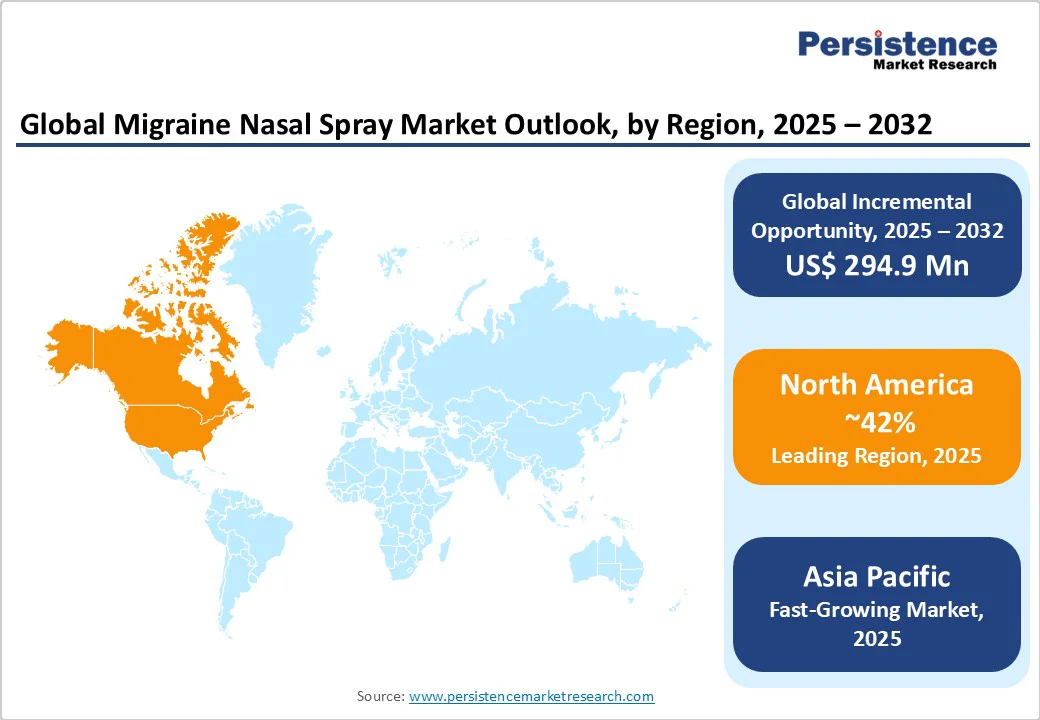
North America is projected to account for nearly 42% of the global Migraine Nasal Spray Market in 2025, driven by advanced healthcare infrastructure and high prevalence of migraines in the U.S. The U.S. market is characterized by robust R&D activities, with companies such as Merck and Thermo Fisher Scientific leading innovations in triptan-based nasal sprays.
The high incidence of migraines, affecting approximately 12% of the U.S. population, coupled with strong healthcare spending, fuels demand for fast-acting treatments. The FDA’s streamlined approval processes for migraine therapies and the increasing availability of over-the-counter nasal sprays in retail pharmacies further drive market growth.
Additionally, the growing adoption of preservative-free and combination formulations, supported by private investments in pharmaceutical startups, positions the U.S. as a leader in market innovation and adoption.
Europe is a significant player in the Migraine Nasal Spray Market, supported by strong healthcare systems and collaborative research initiatives. Leading countries such as Germany, France, and the UK are driving market growth through investments in pharmaceutical innovation and increasing awareness of migraine treatments.
The European Medicines Agency (EMA) supports the development of advanced nasal spray formulations, with companies such as Abcam and Biorbyt focusing on preservative-free and combination therapies. The high prevalence of migraines, particularly in Western Europe, and the growing demand for non-invasive treatment options are key drivers.
Europe’s focus on patient-centric healthcare and sustainable drug development further strengthens its market position, ensuring steady growth in the forecast period.
Asia Pacific is the fastest-growing market for migraine nasal sprays, driven by increasing healthcare access and rising awareness of migraine treatments in countries such as China and India. China’s pharmaceutical industry is expanding rapidly, with companies such as Shanghai Maokang Biotechnology investing in cost-effective nasal spray formulations.
India’s growing healthcare infrastructure and increasing prevalence of migraines, particularly in urban areas, are boosting demand for affordable and accessible treatments. The rise of online pharmacies and government initiatives to improve healthcare access are further accelerating market growth.
Additionally, the adoption of preservative-free formulations and the growing focus on personalized medicine are creating substantial opportunities for market expansion in the region.
The global Migraine Nasal Spray Market is highly competitive, characterized by a mix of global pharmaceutical giants and specialized biotechnology firms. In developed regions such as North America and Europe, large players such as Thermo Fisher Scientific, Merck, and Abcam dominate through advanced R&D capabilities and established distribution networks.
In the Asia Pacific, regional players such as Shanghai Maokang Biotechnology and Vigorous Biotechnology are gaining traction by offering cost-effective solutions tailored to local markets. Companies are focusing on product innovation, such as preservative-free and combination formulations, to gain a competitive edge.
Strategic partnerships, acquisitions, and investments in smart delivery systems are further intensifying the competitive landscape. Leading companies are investing heavily in R&D to develop innovative formulations, such as preservative-free and combination nasal sprays, to meet evolving patient needs.
Strategic collaborations with healthcare providers and online pharmacies are enhancing market reach, particularly in emerging markets. Additionally, companies are leveraging digital marketing and patient education campaigns to increase awareness and adoption of nasal spray treatments.
The global Migraine Nasal Spray Market is projected to reach US$ 495.4 Mn in 2025.
The rising prevalence of migraines and demand for rapid relief are key drivers.
The market is poised to witness a CAGR of 6.9% from 2025 to 2032.
Advancements in preservative-free and combination formulations are a key opportunity.
Thermo Fisher Scientific, Abcam, Merck, Cayman Chemical, and Santa Cruz Biotechnology are key players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Ingredient
By Formulation Type
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author