ID: PMRREP32139| 200 Pages | 14 Aug 2025 | Format: PDF, Excel, PPT* | Healthcare

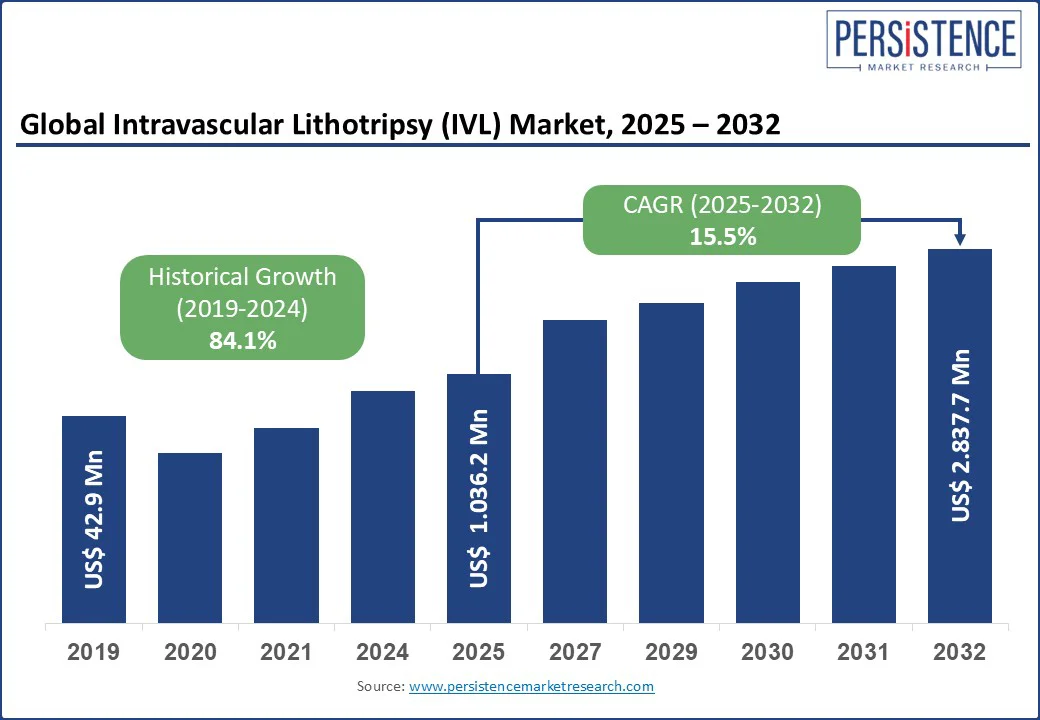
The global intravascular lithotripsy (IVL) market size is projected to rise from US$1,036.2 Mn in 2025 to US$2,837.7 Mn by 2032. It is anticipated to witness a CAGR of 15.5% during the forecast period from 2025 to 2032.
As the prevalence of calcified arterial problems increases with age, there is a high demand forintravascular lithotripsy in treating older patients, who may not be suitable for invasive procedures. The number of people aged 65 and older in the U.S. is projected to reach 80 million by 2040, significantly increasing the pool of patients who could benefit from IVL.
The intravascular lithotripsy (IVL) market is gaining significant traction, particularly in the treatment of coronary artery disease (CAD). IVL, a minimally invasive technique, uses shockwaves to break down calcified plaque in arteries, improving blood flow and allowing for better stent placement. The market is experiencing rapid growth, driven by technological advancements, an aging population, and the rising prevalence of lifestyle-related risk factors such as obesity and diabetes.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Intravascular lithotripsy (IVL) Market Size (2025E) |
US$1,036.2 Mn |
|
Projected Market Value (2032F) |
US$2,837.7 Mn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
15.5% |
|
Historical Market Growth Rate (CAGR 2019 to 2024) |
84.1% |
The increasing prevalence of vascular calcification remains a key driver for the intravascular lithotripsy (IVL) market. Vascular calcification, which is linked to aging and chronic diseases such as diabetes, kidney disease, and hypertension, is becoming common, affecting up to 30-40% of adults over 65 years old. This condition leads to arterial stiffening, heightening the risk of heart attacks, strokes, and other cardiovascular events.
As awareness of the condition’s impact on cardiovascular health rises, so does the demand for effective, minimally invasive treatments such as IVL. IVL technology, which uses shockwaves to break down calcified plaques, offers a safe alternative to traditional methods.
In June 2024, AVS received an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin its pivotal trial for Pulse IVL™. This study evaluated the safety and efficacy of the system in treating U.S.-based patients with severely calcified peripheral arterial disease.
Patient preference for minimally invasive cardiovascular treatments is a significant fueling factor for intravascular lithotripsy (IVL) market growth, particularly for procedures such as intravascular lithotripsy (IVL). These treatments offer numerous benefits over traditional methods, including shorter recovery times, reduced risks of complications, and minimal discomfort.
As patients become more informed about their healthcare options, there is a growing demand for less invasive procedures that offer fast healing and few post-operative restrictions. This trend is especially prominent among elderly patients and those with comorbidities, who may be at higher risk for complications with open surgeries.
The shift toward minimally invasive treatments are further supported by advancements in medical technology, improving both safety and efficacy. Consequently, the growing preference for these options is propelling the expansion of the cardiovascular treatment market, with continued innovation and patient-centric care driving adoption rates. This trend is expected to sustain its momentum as healthcare providers prioritize patient comfort and quicker recovery times in their treatment protocols.
The high costs of intravascular lithotripsy (IVL) devices represent a significant restraint in expanding their accessibility. Due to the advanced technology and precision required, these devices come with a high price tag, which makes them unaffordable for many healthcare facilities, especially those in developing regions or those with limited resources.
The cost barrier is passed on to patients, which may result in increased out-of-pocket expenses. Additionally, insurance coverage for IVL treatments may be limited, further hindering access for many individuals. As a result, the high costs of IVL devices remain a key challenge for broad market adoption and treatment accessibility.
Emerging healthcare markets present significant opportunities in the intravascular lithotripsy (IVL) market. As healthcare infrastructure improves in developing regions, there is increasing access to advanced medical technologies, which drives the demand for innovative treatments such as IVL.
Rising awareness of cardiovascular diseases and the growing burden of conditions such as vascular calcification in these markets further contribute to the adoption of minimally invasive procedures. Healthcare providers in these regions are prioritizing cost-effective, patient-friendly treatments that offer quick recovery times. These factors create a favorable environment for the expansion of IVL technologies, making emerging healthcare markets key growth areas for the industry.
The integration of imaging modalities, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), with intravascular lithotripsy (IVL) procedures enhances precision and outcomes. These imaging techniques allow for real-time visualization of calcified plaques, improving lesion assessment and treatment planning. By providing detailed, high-resolution images, they guide the accurate placement of IVL catheters, ensuring targeted shockwave delivery.
Integration minimizes procedural complications, reduces the need for repeat interventions, and enhances patient safety. As IVL technology continues to evolve, synergy with imaging modalities will contribute to more effective treatments, driving adoption and improving clinical outcomes in cardiovascular care.
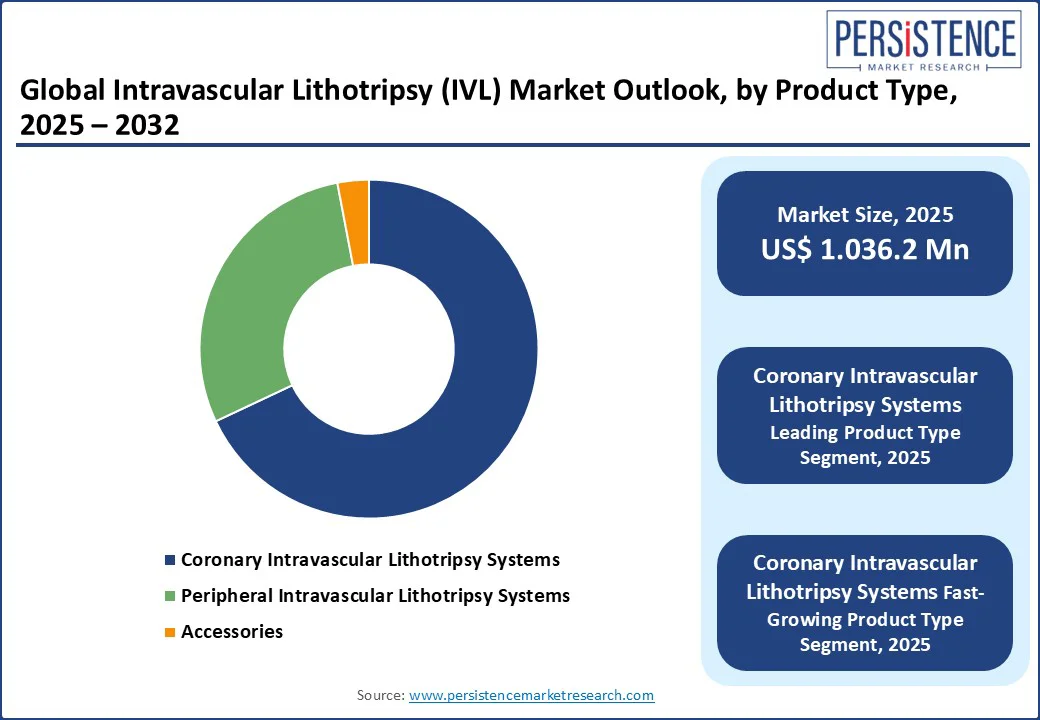
The coronary intravascular lithotripsy systems segment maintains a dominant position in the market, accounting for 69.3% of the share in 2025. Growth is attributed to their effectiveness in treating complex calcified coronary lesions, which are often difficult to address with traditional angioplasty techniques.
IVL systems follow focused shockwaves to break down calcium buildup, improving vessel compliance and enabling better stent placement. With a rising prevalence of coronary artery disease (CAD) and an increasing demand for minimally invasive treatments, coronary IVL systems are expected to maintain strong market demand. Consequently, driving innovation and expanding market share among leading manufacturers.
The coronary artery disease segment maintains primacy in the market, accounting for a substantial 60.4% market share in 2025. The growth is due to rising incidences of lifestyle-related factors. such as obesity, smoking, and sedentary habits, contribute to the growing CAD patient population. Advanced diagnostic and therapeutic technologies, including minimally invasive procedures, play a crucial role in addressing this burden.
Increasing awareness about early diagnosis and proactive management of CAD is fostering segment growth. Favorable reimbursement policies and strategic investments in innovative treatment options, such as intravascular lithotripsy (IVL), enhance patient outcomes, reinforcing the dominance. Key players focus on expanding their product portfolios to sustain leadership.
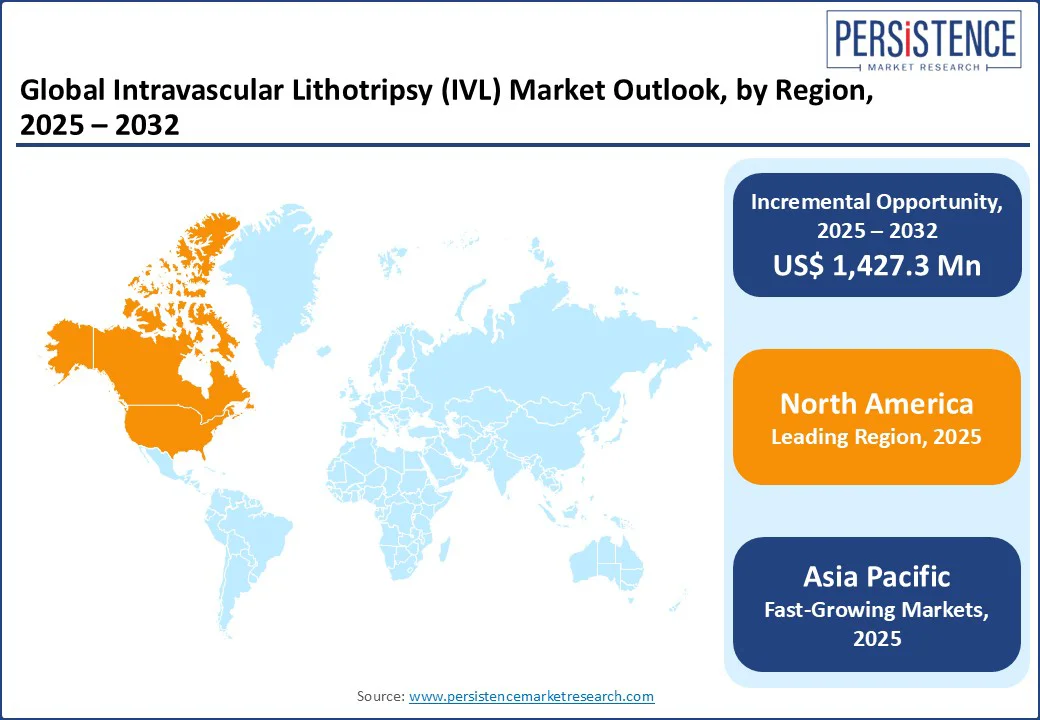
North America generated a significant share of 79.8% in 2025, fueled by strong healthcare infrastructure, high levels of awareness, and favorable reimbursement policies. The increasing adoption of IVL systems in treating complex calcified lesions ensures effective patient outcomes, fueling market growth.
The region also benefits from robust healthcare infrastructure, high healthcare spending, and strong regulatory support, particularly in the U.S. Moreover, ongoing clinical trials and strategic collaborations among key players solidify the region’s position, fostering innovation and extending accessibility across North America.
The global intravascular lithotripsy market is characterized by rapid technological innovations, with companies focusing on improving the safety and effectiveness of their devices. Partnerships, acquisitions, and regulatory approvals are also vital growth strategies.
Johnson & Johnson’s Shockwave devices lead the global IVL market. Shockwave has been the only available IVL system for almost eight years, giving it a strong edge. However, this lead is now being challenged. In January 2025, Boston Scientific fully acquired Bolt Medical. Bolt’s IVL device got FDA approval in mid-2025, allowing Boston Scientific to directly compete with Shockwave. Abbott is also showing interest in the IVL space. The company plans to start clinical trials for its own IVL technology soon.
Emerging players are exploring opportunities in underserved regions, while established players continue to expand their presence globally. Ongoing clinical trials and FDA approvals, like that of AVS's Pulse IVL™, further impel market dynamics.
The intravascular lithotripsy (IVL) market is estimated to increase from US$1,036.2 Mn in 2025 to US$2,837.7 Mn in 2032.
The global intravascular lithotripsy (IVL) market growth is driven by advanced IVL catheters that facilitate access to complex lesions, the emerging application in Below-the-Knee (BTK) arterial revascularization, and industry consolidation through strategic high-value acquisitions.
The intravascular lithotripsy (IVL) market is projected to record a CAGR of 15.5% during the forecast period from 2025 to 2032.
Opportunities include growing adoption of intravascular lithotripsy (IVL) in emerging healthcare markets, development of lower‑cost, portable systems, and expanding PAD/CAD applications.
Major players include Aste Shockwave® Medical Inc. (Johnson & Johnson Services, Inc.), Bolt Medical, Inc. (Boston Scientific), Cardiovascular Systems, Inc. (Abbott), and AVS Pulse, Inc.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2025 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Disease Indication
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author