ID: PMRREP4185| 175 Pages | 7 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

The global herpes markers testing market size is likely to value US$ 616.7 million in 2025 and is projected to reach US$ 927.3 million by 2032, growing at a CAGR of 6.0% between 2025 and 2032.
Herpes marker testing is an essential diagnostic tool used for detecting infections caused by the herpes simplex virus (HSV), specifically HSV-1 and HSV-2. HSV-1 typically leads to orofacial conditions, such as cold sores, whereas HSV-2 primarily causes genital herpes.
Accurate detection of these infections is critical for effective disease management and prevention of complications. Diagnostic solutions include HSV-1 kits, HSV-2 kits, and combined HSV-1/HSV-2 kits, catering to diverse clinical needs.
| Key Insights | Details |
|---|---|
| Global Herpes Markers Testing Market Size (2025E) | US$ 616.7 million |
| Market Value Forecast (2032F) | US$ 927.3 million |
| Projected Growth (CAGR 2025 to 2032) | 6.0% |
| Historical Market Growth (CAGR 2019 to 2024) | 5.6% |
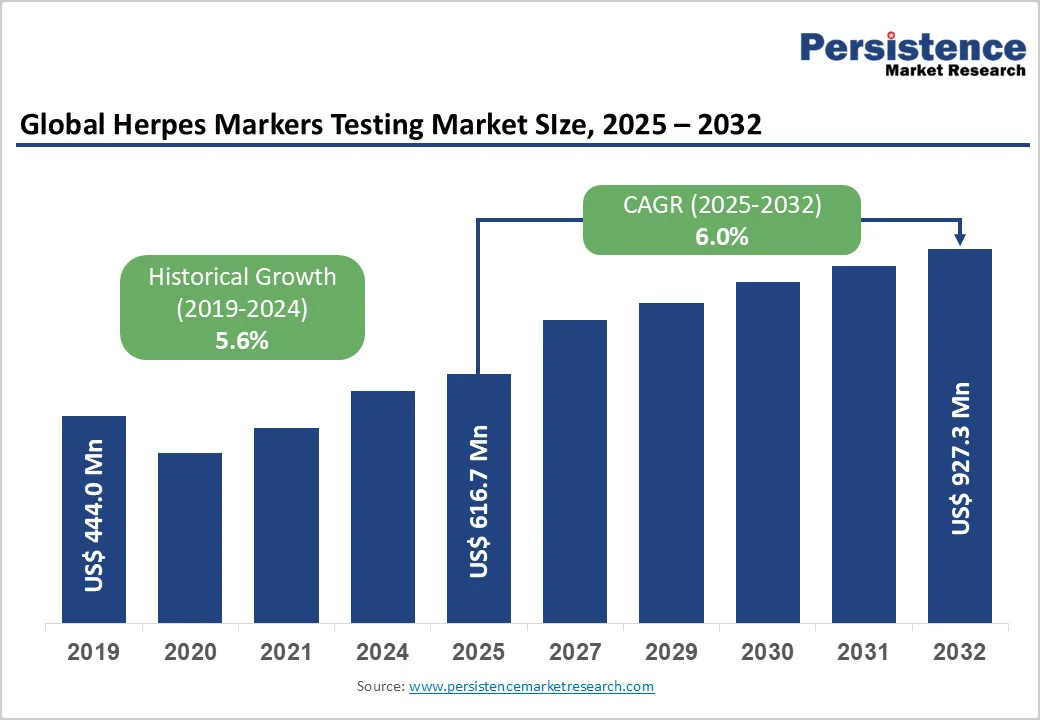
The global herpes markers testing market is witnessing steady expansion, driven by the high global prevalence of herpes simplex virus (HSV) infections, increasing disease awareness, growing healthcare expenditure, and continuous technological advancements.
According to the World Health Organization (WHO, 2025), nearly 3.8 billion people under 50 (64%) are infected with HSV-1, while 520 million adults aged 15-49 (13%) carry HSV-2-the leading cause of genital herpes. HSV infections often remain asymptomatic, yet symptomatic genital herpes affects over 205 million people globally, posing significant clinical and economic burdens. HSV-2 infections notably triple the risk of HIV acquisition, reinforcing the need for accurate and accessible testing solutions.
The market is further fueled by ongoing government and global health initiatives-such as WHO’s Global Health Sector Strategy (2022 - 2030) and the National Institute of Health (NIH)-supported STI (sexually transmitted infection) Watch portal-aimed at improving STI surveillance and vaccine development.
Parallelly, the emergence of new molecular diagnostic technologies and ongoing clinical trials for both preventive and therapeutic HSV vaccines are creating robust opportunities for innovation in herpes marker diagnostics worldwide.
The herpes markers testing market faces several restraints that hinder its overall growth despite rising disease prevalence. Limited access to reliable diagnostic services in remote and low-resource regions continues to delay early detection and treatment, contributing to disease transmission and underreporting.
Economic and regulatory challenges also weigh heavily. Recent studies show the global costs associated with genital herpes amount to an estimated USD 35 billion annually, reflecting both healthcare expenditure and productivity losses.
Additionally, diagnostic accuracy concerns have impacted confidence in certain testing methods. In 2023, the U.S. FDA (Food and Drug Administration) cautioned against false-positive HSV-2 serological results, urging confirmatory testing to minimize misdiagnosis and patient distress.
Following similar reasoning, Alverno Laboratories discontinued HSV IgM antibody testing in 2024 in alignment with CDC guidelines, citing its non-non-type-specific nature and low clinical reliability. Alongside high costs, limited R&D investment, and regulatory barriers, these factors collectively restrain the pace of innovation and adoption within the global herpes marker testing market.
The herpes markers testing market presents significant growth opportunities driven by ongoing research breakthroughs, emerging antiviral therapies, and increasing integration of molecular diagnostics in infectious disease management.
In August 2025, Assembly Biosciences announced advancement of its investigational HSV drug, ABI-5366, into Phase II clinical trials after demonstrating an impressive 94% reduction in viral shedding during its Phase Ib study (NCT06385327).
This long-acting HSV helicase-primase inhibitor also showed a 94% reduction in genital lesion rate compared to placebo, far exceeding clinical expectations. Such progress underscores the growing clinical emphasis on precision medicine and early-stage disease monitoring, both of which rely on advanced diagnostic tools.
Additionally, the expansion of point-of-care and multiplex PCR platforms offers immense potential for real-time detection and differentiation of HSV-1 and HSV-2 infections. Collaborations between pharmaceutical innovators and diagnostic developers are further expected to create integrated testing-treatment models, facilitating personalized care and improving patient outcomes-thus positioning herpes markers testing as a critical component of next-generation infectious disease management.
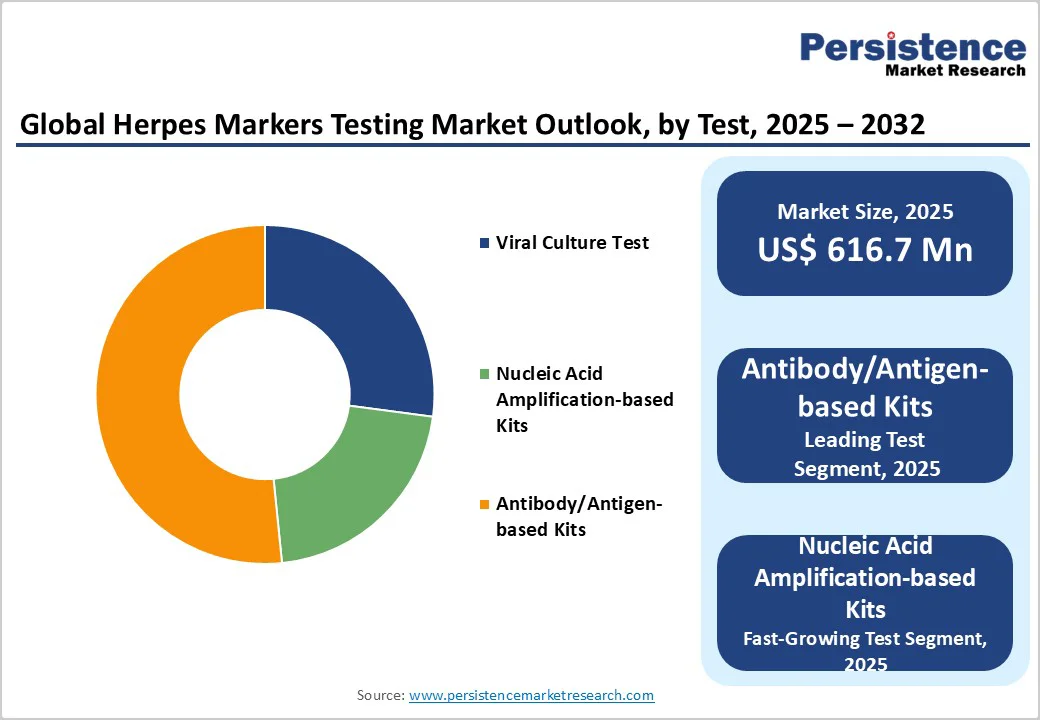
The antibody and antigen-based kits segment is projected to dominate the herpes marker testing market, holding approximately 51.6% value share throughout the forecast period. This segment’s leadership is driven by wide product availability and strong physician preference for these reliable, well-established testing methods.
In contrast, nucleic acid amplification-based kits, particularly PCR-based tests, are expected to be the fastest-growing segment due to increasing adoption in clinical settings. PCR tests offer high sensitivity and specificity, rapid turnaround times, and the ability to accurately differentiate between HSV-1 and HSV-2, making them increasingly preferred for early and precise diagnosis.
The HSV-1/HSV-2 segment is projected to remain the leading indication in the herpes marker testing market in 2025, accounting for approximately 49.4% of the global market share. This dominance is attributed to the increasing clinical need to differentiate between HSV-1 and HSV-2 infections in patients presenting with oral or genital lesions, as co-testing provides a comprehensive diagnostic overview.
Combining the detection of both virus types helps guide appropriate treatment decisions, informs patient counseling, and reduces misdiagnosis. Additionally, the growing prevalence of asymptomatic infections and recurrent genital herpes cases further reinforces the demand for dual-detection assays.
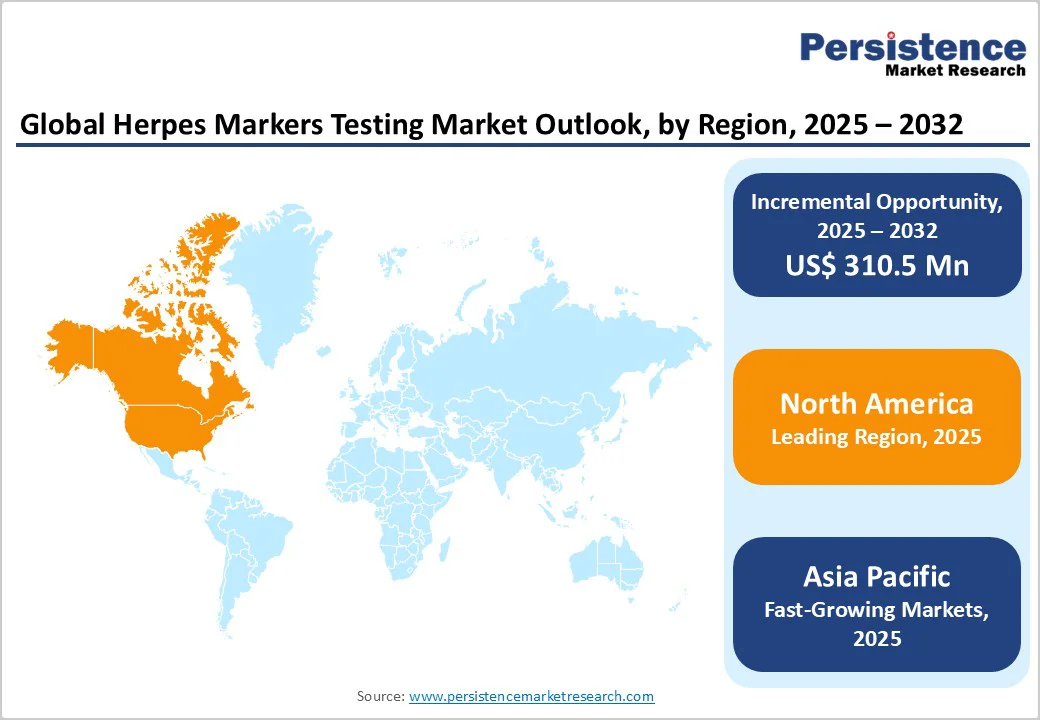
The North America herpes markers testing market is expanding rapidly and is projected to account for approximately 32.1% of the global market share by 2025. In the United States, an estimated 65 million people are living with genital herpes, yet nearly 90% remain undiagnosed, highlighting a critical need for faster, more accurate, and accessible screening and confirmation technologies.
Traditional ELISA tests often produce 17-22% equivocal results, and the Centers for Disease Control and Prevention’s (CDC) Herpes - STI Treatment Guidelines report shows specificity as low as 39.8% for index values of 1.1-2.9, contributing to misdiagnosis, patient distress, and delayed care. Point-of-care solutions like the BioCard™ HSV-2 Rapid Test, launched in July 2025, address these gaps by providing accurate, simple, and scalable testing in clinical and community settings.
CDC estimates further indicate that roughly 20% of the U.S. population has an STI, with approximately 300,000 new genital herpes infections diagnosed annually (CDC, 2024). Beyond morbidity, HSV-2 infection increases HIV acquisition risk by two- to threefold and poses serious risks such as neonatal herpes.
In July 2025, the U.S. Department of Health and Human Services updated its Guidelines for the Prevention and Treatment of Opportunistic Infections, enhancing HSV diagnostic and treatment recommendations, revising immunization schedules, and expanding guidance on related infectious diseases, further supporting testing adoption and improved patient outcomes.
The European herpes markers testing market is experiencing notable growth, driven by evolving epidemiological trends and increasing healthcare awareness, and is projected to hold approximately 25.7% of the total market share by 2025.
A systematic review and meta-analysis of HSV-1 prevalence in Europe revealed a pooled mean seroprevalence of 67.4%, with a steady decline of 1% per year. Notably, HSV-1 is increasingly detected in genital herpes cases, accounting for 34.1% of such diagnoses, a trend that has been rising annually by 1%. This shift underscores the need for accurate diagnostic tools and effective treatment strategies.
Furthermore, the economic burden of herpes infections is substantial, with global costs reaching approximately $35.3 billion (€32.9 billion) annually. In Europe, this economic impact is significant, highlighting the importance of efficient diagnostic and treatment options to mitigate healthcare expenditures and productivity losses.
The convergence of high disease prevalence and economic implications presents a compelling case for the expansion and advancement of herpes marker testing in Europe, aiming to enhance patient outcomes and reduce the overall healthcare burden.
Asia Pacific is witnessing rapid growth with CAGR of 9.5%, over the forecast period, driven by increasing awareness of sexually transmitted infections (STIs), expanding healthcare infrastructure, and rising infection rates.
Countries such as China, India, and Japan are enhancing screening efforts through public health campaigns, prenatal care programs, and digital healthcare adoption. In China, researchers have identified genes that help protect against severe herpes complications, supporting improved diagnostic and therapeutic strategies.
Home-based HSV-2 testing is also being introduced in parts of Central Asia, enabling early detection and prevention of transmission. Regional initiatives, such as WHO programs in the Maldives targeting maternal and child health, underscore the emphasis on infection control and early screening. Technological advancements, including the launch of new RT-PCR detection kits and rapid testing platforms, are improving test sensitivity and accessibility.
Despite challenges like limited healthcare services in remote areas, increasing sexual health awareness, government support, and integration of advanced diagnostic tools are collectively driving the expansion of herpes markers testing across the Asia Pacific.
The global herpes marker testing market features a wide range of regional and international players. Companies are actively pursuing product launches, regulatory approvals, and technology innovations to expand their presence.
Strategic initiatives, including collaborations, licensing agreements, and showcasing new diagnostic platforms, are increasingly employed to reach broader clinical and public health markets, enhance early detection, and improve management of HSV-1 and HSV-2 infections across diverse geographies.
The global herpes markers testing market is projected to be valued at US$ 616.7 million in 2025.
Rising prevalence of herpes infections, increasing awareness, and growing demand for early and accurate diagnostics drive the global market.
The global market is poised to witness a CAGR of 6.0% between 2025 and 2032.
Expansion of point-of-care testing, integration with routine STI screening, and adoption of advanced molecular diagnostic technologies offer lucrative opportunities.
Major players in the global are AdvaCare Pharma, Quest Consumer Inc., Abbott, Thermo Fisher Scientific Inc., Roche Diagnostics GmbH, Labcorp and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Test
By Indication
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author