ID: PMRREP32774| 176 Pages | 16 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

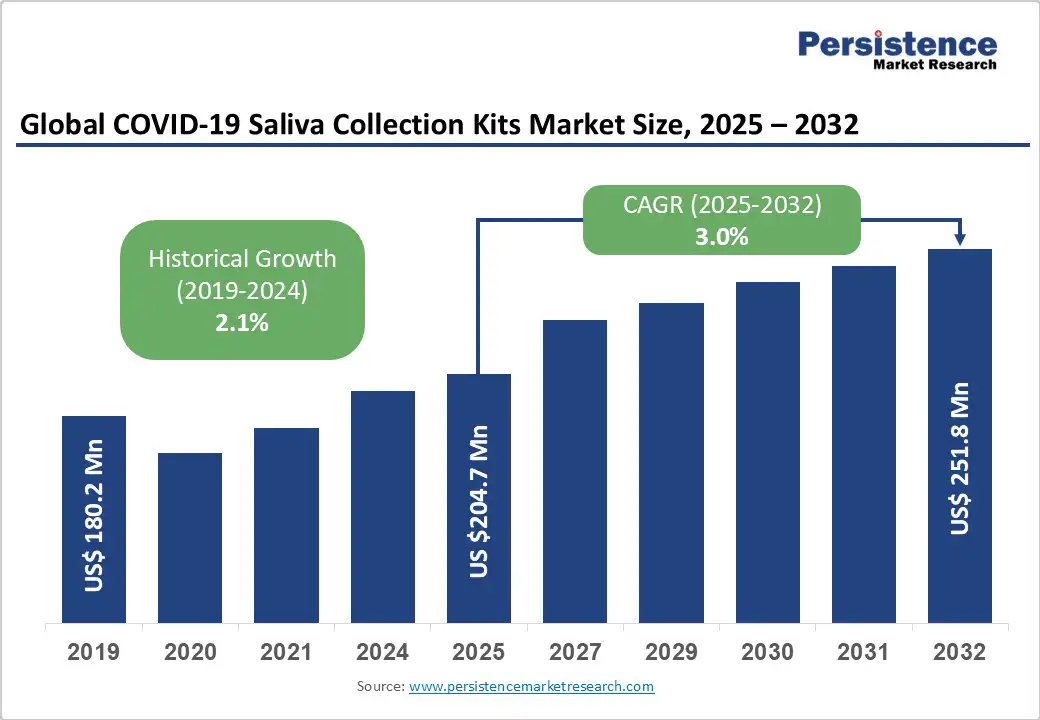
The global COVID-19 saliva collection kits market size is likely to be valued at US$204.7 Million in 2025 and is estimated to reach US$251.8 Million in 2032, growing at a CAGR of 3.0% during the forecast period 2025 - 2032, driven by the ease of sample collection, which encourages frequent testing, especially in schools, workplaces, and at-home settings.
| Key Insights | Details |
|---|---|
| COVID-19 Saliva Collection Kits Market Size (2025E) | US$204.7 Mn |
| Market Value Forecast (2032F) | US$251.8 Mn |
| Projected Growth (CAGR 2025 to 2032) | 3.0% |
| Historical Market Growth (CAGR 2019 to 2024) | 2.1% |
A major driver for the adoption of COVID-19 saliva collection kits is their non-invasive and painless nature. Unlike nasopharyngeal swabs, which can cause discomfort and coughing, saliva collection is simple, stress-free, and suitable for repeated testing, including among children and the elderly. For example, Yale’s SalivaDirect program demonstrated that participants preferred saliva tests over traditional swabs due to ease of use and minimal discomfort.
This convenience has made saliva kits attractive for schools, workplaces, and large-scale surveillance programs. By reducing resistance to testing, non-invasive collection increases compliance and enables frequent monitoring, a key factor in managing outbreaks and detecting infections early.
COVID-19 saliva collection kits benefit from reducing the risk of virus transmission during testing. Healthcare workers face low exposure risk because patients can self-collect saliva samples without close contact, unlike nasopharyngeal swabs that require trained personnel. Helix and Labcorp have used this advantage in their home collection kits, allowing individuals to provide samples safely and ship them to laboratories without in-person interactions.
This feature is mainly important in high-transmission settings or for vulnerable populations. By lowering infection risk for healthcare workers and communities, saliva-based testing supports safe large-scale screening and strengthens public confidence in diagnostic measures.
A key challenge limiting market growth is the comparatively lower sensitivity in certain cases, which can lead to false-negative results. While saliva tests are convenient and non-invasive, studies have shown that their accuracy can be slightly lower than nasopharyngeal swabs, especially for asymptomatic individuals or early-stage infections.
For instance, research from Yale University’s SalivaDirect program noted that while saliva-based PCR tests are reliable, they occasionally miss low viral loads that standard swab tests detect. This limitation impacts the confidence of healthcare providers and public health authorities in relying solely on saliva tests, specifically in critical diagnostic or clinical trial settings.
Another growth barrier is the natural variability in viral load present in saliva samples. Viral concentrations can fluctuate throughout the day or based on factors such as food intake, hydration, and oral hygiene. This inconsistency can lead to inaccurate or inconclusive test results, reducing reliability for large-scale screening.
For example, a study published in The Lancet Microbe showed that some individuals with COVID-19 exhibited detectable virus in nasal swabs but not in saliva at certain times, complicating test interpretation. Such variability necessitates repeated sampling or confirmatory testing, which can increase costs, reduce convenience, and slow adoption.
COVID-19 saliva collection kits are seeing growth opportunities through the development of improved diagnostic assays. Companies are investing in assays that increase sensitivity and accuracy, especially for detecting low viral loads and emerging variants. For example, U.S.-based Lucira Health is working on next-generation saliva-based PCR tests capable of detecting multiple respiratory pathogens in addition to COVID-19, enabling multiplexed testing.
Researchers in Japan have further developed RT-LAMP-based assays for saliva that provide fast results without compromising reliability. These innovations make saliva testing more versatile, allowing laboratories and healthcare providers to expand beyond COVID-19 diagnostics, tapping into broad infectious disease detection markets. The focus on improved assay performance positions saliva kits as a durable diagnostic tool, even as pandemic waves subside.
Another significant growth avenue is the simplification of home-based saliva sample collection. The demand for convenient, non-invasive, at-home testing solutions continues to rise, especially in remote or high-risk communities. Helix has already introduced home saliva kits with easy-to-follow instructions, pre-paid shipping, and rapid lab processing, making testing accessible without clinic visits.
In Singapore, initiatives allowing residents to submit self-collected saliva samples for national surveillance have demonstrated the efficiency and scalability of home-based collection. By reducing the reliance on healthcare personnel and specialized equipment, simplified home collection refines adoption, improves compliance, and creates opportunities in public health monitoring, school and workplace screening, and routine infectious disease testing.
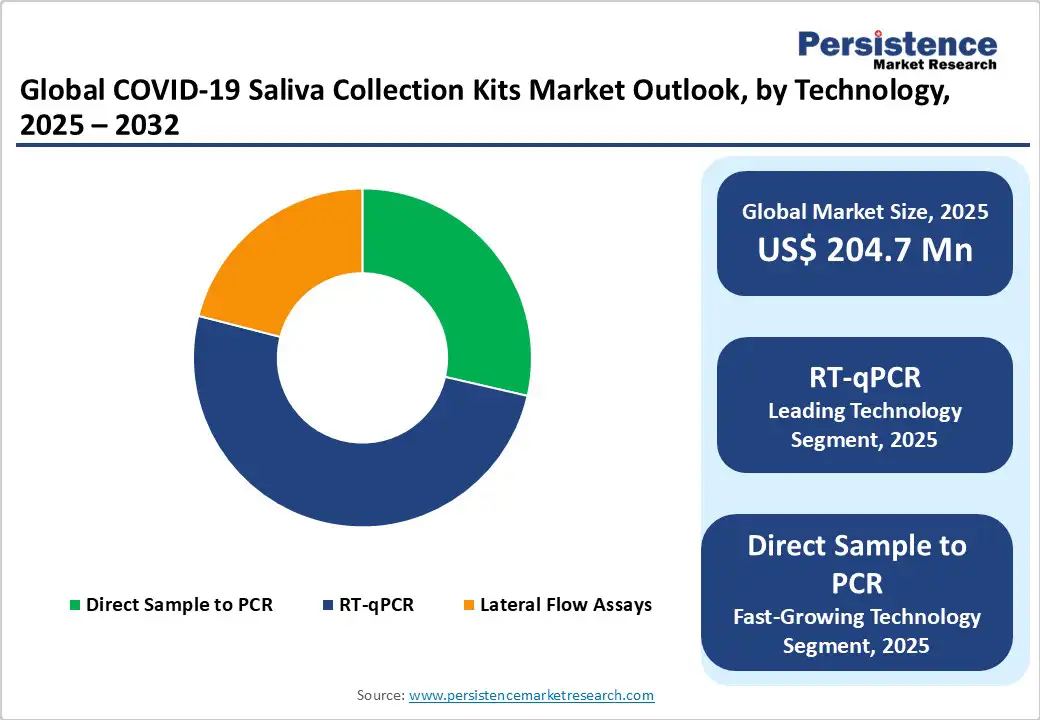
PCR-based kits are expected to account for nearly 71.4% of the market share in 2025, owing to their high sensitivity and reliability in detecting the virus, even at low viral loads. They can identify asymptomatic infections and early-stage cases, making them important for accurate diagnostics. Laboratories and healthcare providers favor these kits for clinical settings and research studies, backed by their reproducibility and validated accuracy.
Rapid test kits, including antigen and lateral flow assays using saliva, are experiencing steady growth due to their quick turnaround time and ease of use outside laboratory settings. These kits deliver results in minutes, making them ideal for at-home testing, airports, schools, and workplaces. For instance, Abbott’s BinaxNOW COVID-19 test has been widely adopted for point-of-care screening, providing results in 15 minutes.
RT-qPCR is speculated to capture a share of around 50.4% in 2025, spurred by its unmatched sensitivity and precision in detecting viral RNA. It can quantify viral load in real time, making it highly reliable for diagnosing asymptomatic and early-stage infections. The technology’s superiority and reproducibility make it a standard choice for clinical laboratories, research studies, and public health monitoring, ensuring confidence in test outcomes even as testing demands fluctuate.
Direct sample-to-PCR technology improves COVID-19 testing by eliminating RNA extraction steps, reducing processing time, costs, and potential errors. Saliva samples can be directly introduced into PCR assays without complex lab preparation, enabling quick and high-throughput testing. For instance, studies on modified SalivaDirect workflows have shown that direct PCR maintains sensitivity while cutting turnaround times by several hours.
Diagnostic laboratories are projected to hold a share of approximately 31.8% in 2025 as they possess the infrastructure, skilled personnel, and equipment necessary for high-throughput PCR and RT-qPCR testing. These labs handle large volumes of samples efficiently, ensuring rapid and accurate results for population-level surveillance and research studies.
Hospitals and clinics are key users of COVID-19 saliva collection kits due to their role in patient care and infection control. Saliva-based tests deliver a non-invasive, low-risk alternative to nasopharyngeal swabs, which is mainly beneficial for pediatric, elderly, and immunocompromised patients.
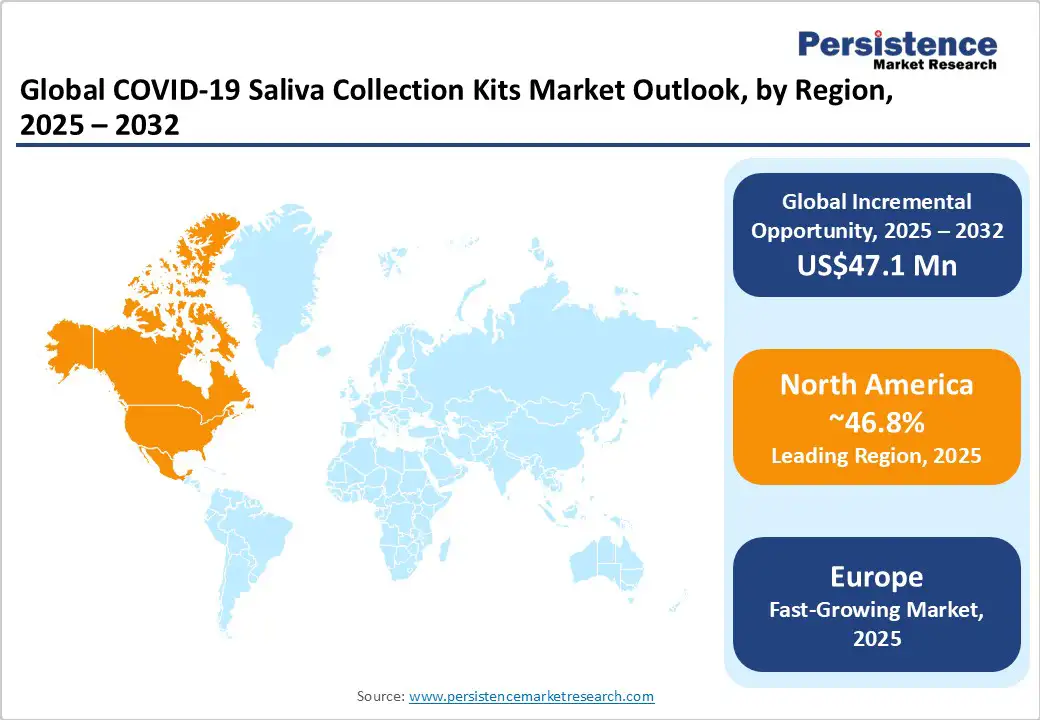
North America is estimated to account for approximately 46.8% of the market share in 2025, as COVID-19 saliva collection kits have become a central component of pandemic response strategies. In the U.S., the Food and Drug Administration (FDA) granted emergency use authorization to Yale's SalivaDirect test in August 2020, marking a milestone in saliva-based diagnostics. The test, which processes saliva samples quickly and is less expensive and invasive compared to traditional methods, does not require specific swabs or collection devices, preventing the shortage of medical supplies.
The U.S. government's Biomedical Advanced Research and Development Authority (BARDA) has been instrumental in supporting the development and distribution of COVID-19 diagnostics, including saliva-based tests. BARDA's involvement has facilitated the fast deployment of these tests across the country. The researchers at the University of Texas have developed a portable ‘lab-on-a-chip’ device capable of differentiating between COVID-19 and the flu using saliva samples.
In Europe, COVID-19 saliva collection kits have gained traction due to their non-invasive nature and ease of use. The U.K. is seeing a surging emphasis on home-based diagnostic kits bearing CE marks. The European Centre for Disease Prevention and Control (ECDC) has acknowledged that saliva-based RT-PCR tests deliver comparable sensitivity to nasopharyngeal swabs for symptomatic cases, exhibiting their reliability in diagnostic settings.
In Spain, the region of Galicia pioneered the use of saliva-based testing, enabling individuals to collect samples at home and send them for analysis. This approach, which processes up to 100,000 tests per month, has been beneficial for the elderly population. The European Union has also established a common list of COVID-19 antigen tests, including those utilizing saliva samples, provided they meet defined performance requirements and are validated through independent studies in at least one EU Member State.
In Asia Pacific, the adoption of COVID-19 saliva collection kits has been marked by innovative approaches and regulatory developments. Singapore's Health Sciences Authority approved a novel COVID-19 test in December 2020 that utilizes saliva collected from deep within the throat, improving detection capabilities and facilitating safe, non-invasive testing methods.
In New Zealand, the Ministry of Health has endorsed the use of saliva samples tested by Nucleic Acid Amplification Testing (NAAT) as an equivalent alternative to nasopharyngeal swabs for diagnosing COVID-19. This policy shift underscores the country's commitment to integrating saliva-based diagnostics into its national testing strategy.
The global COVID-19 saliva collection kits market is marked by a shift from emergency response to strategic consolidation and development. Thermo Fisher Scientific, Neogen Corporation, Sarstedt AG&Co.KG, Autogen Inc., and Abbott Laboratories are actively involved in the development and distribution of saliva collection kits.
For instance, Thermo Fisher Scientific's acquisition of Mesa Biotech in 2021 enabled the company to improve its molecular diagnostics capabilities, including saliva-based testing solutions. Abbott Laboratories has also been broadening its portfolio to include non-invasive testing methods, catering to the high demand for at-home and point-of-care diagnostics.
Key business strategies for providers in the COVID-19 saliva collection kits market include expanding product portfolios to cover PCR and rapid tests, as well as forming strategic partnerships and acquisitions. A few other companies are targeting emerging and high-demand markets, investing in research for non-invasive and rapid diagnostics, improving distribution networks, and focusing on at-home and point-of-care solutions to capture evolving demand.
The COVID-19 saliva collection kits market is projected to reach US$204.7 Million in 2025.
Non-invasive collection and reduced transmission risk are the key market drivers.
The COVID-19 saliva collection kits market is poised to witness a CAGR of 3.0% from 2025 to 2032.
The development of new diagnostic assays and simplified home collection methods represents a key market opportunity.
Qiagen, Thermo Fisher Scientific, and Takara Bio Inc. are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Technology
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author