ID: PMRREP32730| 183 Pages | 18 Aug 2025 | Format: PDF, Excel, PPT* | Healthcare

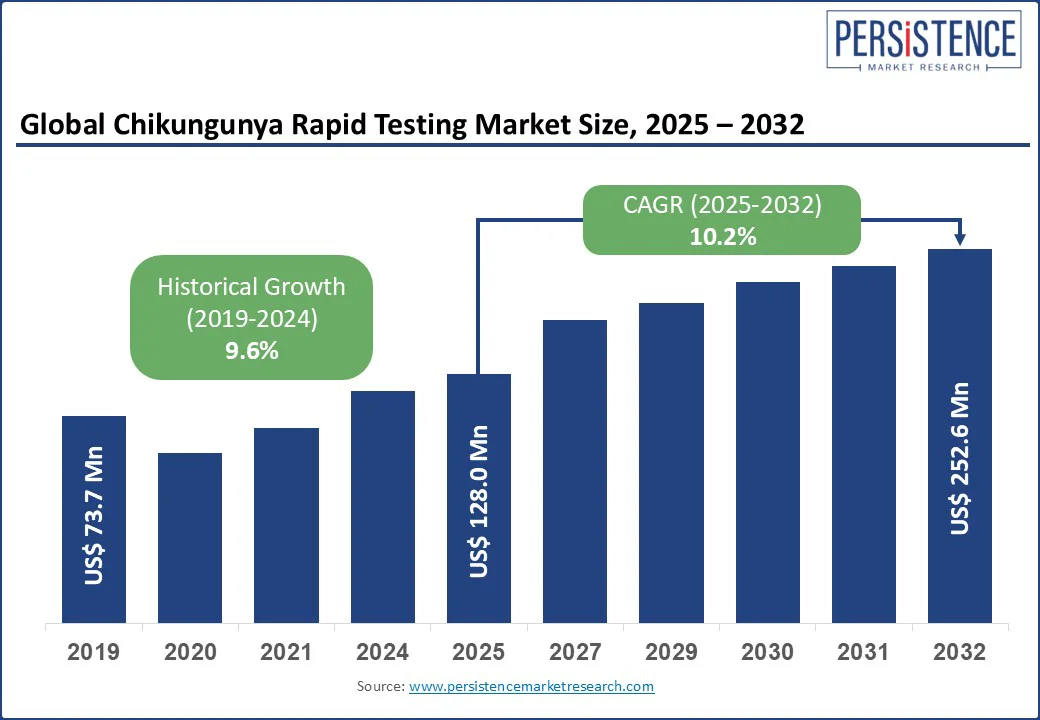
The global Chikungunya Rapid Testing Market size is likely to be valued at US$ 128.0 Mn in 2025 to US$ 252.6 Mn by 2032, registering a CAGR of 10.2% during the forecast period 2025 - 2032.
The chikungunya rapid testing industry has experienced robust growth, driven by the rising prevalence of vector-borne diseases, advancements in diagnostic technologies, and increasing demand for point-of-care testing solutions. The Chikungunya Rapid Testing market's expansion is further supported by global efforts to combat infectious diseases and improve healthcare access in developing regions.
Key Industry Highlights:
Global Market Attribute
|
Key Insights |
Details |
|
Chikungunya Rapid Testing Market Size (2025E) |
US$ 128.0 Mn |
|
Market Value Forecast (2032F) |
US$ 252.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
10.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
9.6% |
The global surge in vector-borne diseases is a primary driver of the chikungunya rapid testing market. According to the World Health Organization, vector-borne diseases account for over 700,000 deaths annually, with chikungunya outbreaks significantly impacting tropical and subtropical regions. In 2024, the Pan American Health Organization reported over 400,000 chikungunya cases in the Americas alone, underscoring the urgent need for rapid diagnostics. The increasing incidence of chikungunya, particularly in densely populated areas, drives demand for efficient and accurate testing solutions.
Technological advancements in rapid diagnostic kits are significantly boosting market growth. Modern systems, such as Quest Diagnostics’ rapid antigen test kits, offer high sensitivity and specificity, reducing false negatives and enabling faster diagnosis. A study published in The Lancet found that automated rapid tests reduced diagnostic turnaround time compared to traditional laboratory methods. Innovations such as portable, user-friendly kits and integration with digital platforms for result reporting further enhance adoption, particularly in resource-limited settings.
Government initiatives and increased funding for infectious disease control are also key growth drivers. In India, programs such as the National Vector Borne Disease Control Programme (NVBDCP) have expanded access to diagnostic testing, increasing demand for rapid kits. In North America, favorable reimbursement policies for diagnostic procedures, coupled with heightened surveillance for imported chikungunya cases, encourage healthcare facilities to invest in advanced testing equipment.
The high cost of chikungunya rapid testing kits remains a significant barrier to widespread adoption, particularly in low- and middle-income countries. Advanced diagnostic kits, equipped with features such as high sensitivity and real-time data integration, require substantial upfront investment. Additionally, ongoing costs for reagents, maintenance, and quality control add to the total cost of ownership.
In regions such as Sub-Saharan Africa and rural parts of South Asia, where healthcare budgets are constrained, these financial burdens limit access to rapid testing, even amidst frequent chikungunya outbreaks. The World Health Organization (WHO) has noted that “the cost of the test may be a disincentive”, even where rapid testing is available. Although this reference is not specific to chikungunya, it highlights high diagnostic costs as a barrier to widespread adoption of rapid tools in many countries
The requirement for skilled personnel to operate and interpret rapid testing systems also hinders market growth. Conducting and analyzing tests, especially molecular-based ones such as PCR, demands specialized training for laboratory technicians. A survey by the Asia Pacific Society of Infectious Diseases reported a shortage of certified diagnosticians in the region’s healthcare facilities. This skills gap, combined with high training costs, restricts the adoption of advanced testing systems in developing markets, slowing market expansion.
The development of portable and compact chikungunya rapid testing kits presents significant growth opportunities, enabling deployment in remote clinics, mobile health units, and emergency outbreak scenarios. These portable systems overcome the limitations of traditional lab-based diagnostics, making them ideal for decentralized healthcare settings.
For example, Inbios India’s portable antigen test kit, launched recently, offers rapid results in under 15 minutes, supporting its use in rural and field settings. As healthcare systems prioritize accessible diagnostics, demand for such solutions is rising, particularly in regions with limited laboratory infrastructure.
The growing popularity of point-of-care (POC) testing, such as lateral flow assays for chikungunya, provides another avenue for market expansion. These tests require minimal equipment and deliver results quickly, making them suitable for resource-constrained environments. A 2024 study in Clinical Infectious Diseases found that POC rapid tests reduced diagnosis-to-treatment time by 35% compared to traditional methods, driving demand for innovative kits in outbreak-prone areas.
The integration of digital health platforms for remote monitoring and data sharing further enhances market potential. Companies such as Bio-Rad Laboratories are incorporating IoT-enabled diagnostics into their testing systems, allowing real-time data transmission and proactive maintenance. This trend improves accessibility and operational efficiency, supporting market growth in both developed and emerging regions.
The global chikungunya rapid testing market is segmented into Antigen Tests, Antibody Tests, and Molecular Tests chikungunya rapid testing market. Antigen Tests dominate, holding approximately 26.35% of the chikungunya rapid testing market share in 2025, due to their critical role in detecting viral proteins during the acute phase of chikungunya infection. Advanced antigen tests, such as Alere’s rapid diagnostic kits, are widely adopted for their ease of use and rapid results, making them essential in outbreak settings.
Molecular Tests are the fastest-growing segment, driven by increasing demand for high-accuracy diagnostics in research and clinical settings. Innovations in nucleic acid amplification technologies, such as Genome Diagnostics’ PCR-based platforms, offer superior sensitivity and specificity, boosting adoption in high-volume diagnostic centers.
The global chikungunya rapid testing market is divided into Rapid Immunochromatographic Tests, Enzyme-Linked Immunosorbent Assay (ELISA), and Polymerase Chain Reaction (PCR). Rapid Immunochromatographic Tests lead with a 46% share in 2025, driven by their widespread use in field and clinical settings. These tests, which deliver results in 10-20 minutes, are critical for managing outbreaks, with over 1 million tests performed annually worldwide.
Polymerase Chain Reaction (PCR) is the fastest-growing segment, fueled by advancements in molecular diagnostics and the rising prevalence of chikungunya. PCR’s ability to confirm infections with high precision drives its adoption in advanced diagnostic facilities, particularly for research and epidemiological studies.
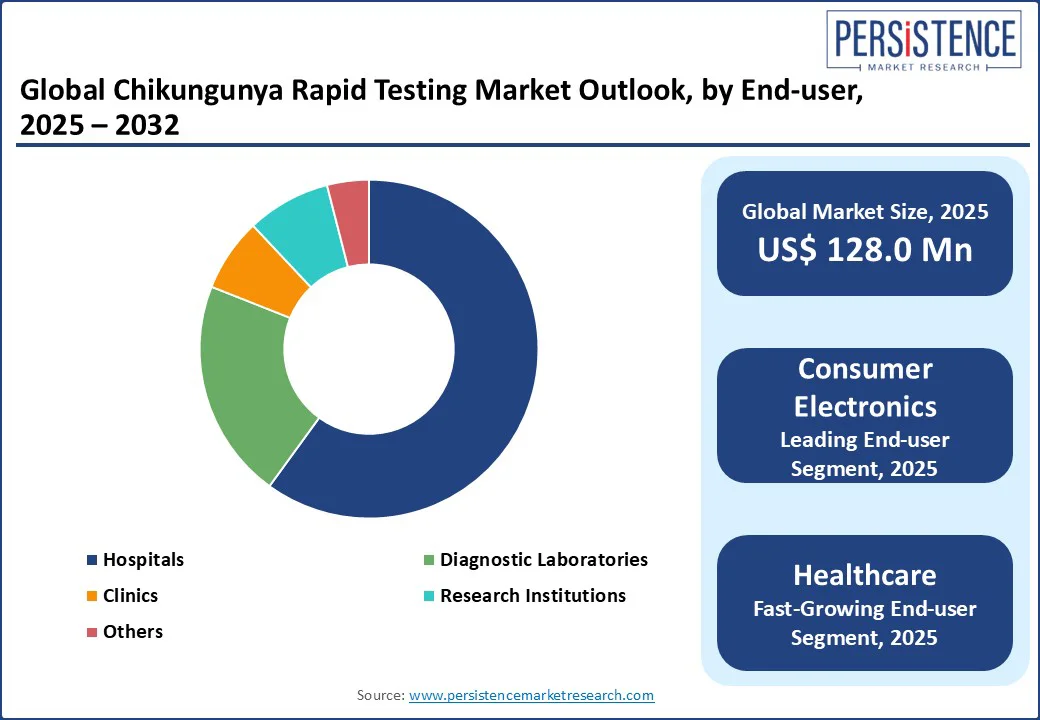
The global chikungunya rapid testing market is segmented into Hospitals, Diagnostic Laboratories, Clinics, and Research Institutions. Hospitals dominate with a 60% share in 2025, driven by their high testing volumes and advanced diagnostic infrastructure. Large hospitals in North America and Europe rely on automated rapid testing systems to handle complex diagnostic needs during outbreaks.
Diagnostic Laboratories are the fastest-growing segment, propelled by the increasing establishment of specialized facilities for infectious disease testing. These labs, equipped with cutting-edge molecular and immunochromatographic technologies, cater to the growing demand for high-throughput testing and research applications.
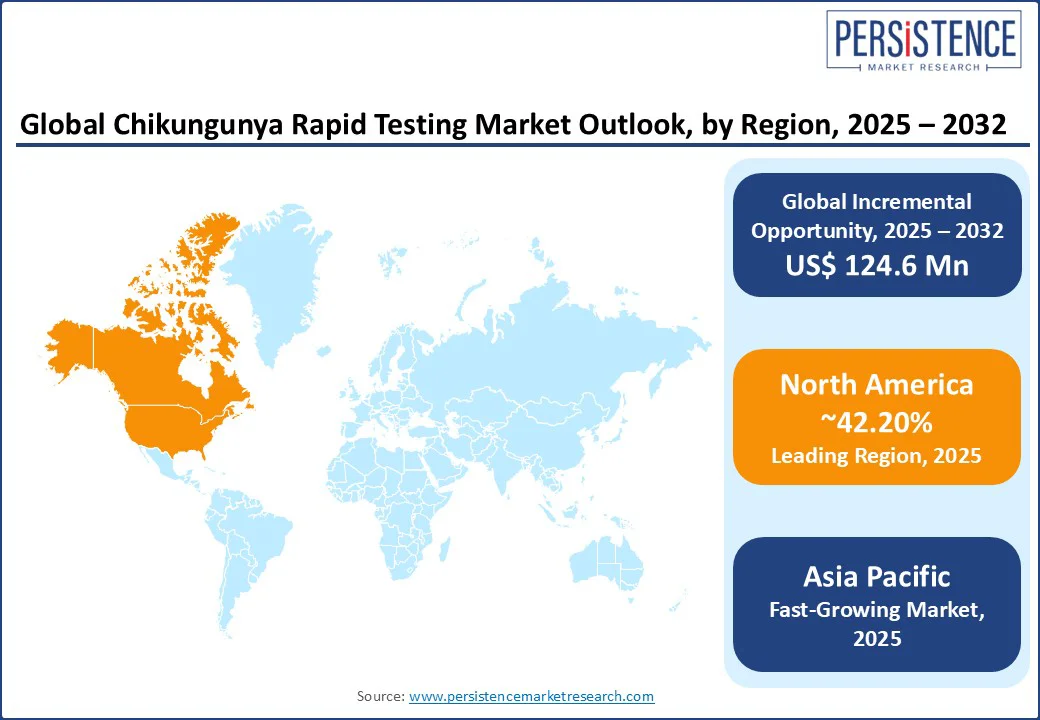
In North America, the U.S. dominates expected to account for 42.20% of the chikungunya rapid testing market share in 2025. This dominance is driven by high surveillance for vector-borne diseases, advanced diagnostic infrastructure, and increasing cases of imported chikungunya. The CDC has reported multiple imported cases annually in recent years, necessitating robust rapid testing solutions. Leading brands such as Quest Diagnostics and Bio-Rad Laboratories offer innovative kits to meet the needs of diagnostic teams.
Consumer preferences are shifting toward compact, automated testing systems with AI-integrated analysis, such as Alere’s rapid antigen kits, which enhance diagnostic accuracy and speed. Stringent FDA regulations prioritize patient safety, encouraging the adoption of reliable, high-sensitivity components. Favorable reimbursement policies for diagnostic tests further incentivize hospitals and labs to invest in advanced equipment, supporting market growth.
Europe’s market is led by Germany, the U.K., and France, driven by regulatory support and high diagnostic volumes. Germany holds the largest share, supported by strong sales from companies such as Sanat Products and Lumiquick Diagnostics. The EU’s Medical Device Regulation (MDR) fosters innovation and compliance, promoting the adoption of advanced antigen and molecular testing systems in major healthcare facilities.
In the U.K., market growth is driven by the rising demand for point-of-care diagnostics, with products such as Etubics Corporation’s rapid kits gaining popularity for their precision and portability. France is witnessing increased demand for outbreak-related diagnostics, with Medical Innovation Ventures (Mediven) offering specialized solutions. Regulatory support for sustainable manufacturing practices across Europe further enhances market prospects.
Asia Pacific represents the fastest-growing market for chikungunya rapid testing, driven by expanding healthcare infrastructure, increasing disease prevalence, and rising investments in diagnostic technologies. India remains a key growth engine, where frequent chikungunya outbreaks and government programs such as the Ayushman Bharat scheme are boosting demand for affordable, semi-automated testing solutions. Domestic manufacturers such as Inbios India cater to local needs with cost-effective kits designed for both urban and rural healthcare settings.
In China, rapid market expansion is supported by large-scale laboratory upgrades, growing adoption of automated rapid testing systems, and the presence of leading players such as CTK Biotech. Japan’s market is characterized by demand for high-precision diagnostic tools used in research and epidemiological surveillance, with companies such as Genome Diagnostics gaining market share. Across the region, increased healthcare spending, digital procurement platforms, and the emphasis on early disease detection are collectively accelerating adoption, making Asia Pacific a critical hub for future market growth.
The global chikungunya rapid testing market is highly competitive, with global and regional players vying for market share through innovation, competitive pricing, and reliability. The rise of portable and automated testing systems intensifies competition, as companies strive to meet stringent regulatory standards and diagnostic demands. Strategic partnerships, mergers, and regulatory approvals are critical differentiators in this dynamic market.
The Chikungunya Rapid Testing market is projected to reach US$ 128.0 Mn in 2025.
Rising vector-borne diseases, technological advancements in rapid diagnostics, and government healthcare initiatives are the key market drivers.
The Chikungunya Rapid Testing market is poised to witness a CAGR of 10.2% from 2025 to 2032.
Innovations in portable testing systems and point-of-care diagnostics present significant growth opportunities.
Quest Diagnostics, Alere, and Genome Diagnostics are among the leading market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Technique Type
By End-user Type
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author