ID: PMRREP35535| 199 Pages | 31 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

The cardiovascular stents market size is likely to be valued at US$ 12.7 Bn in 2025 and is estimated to reach US$ 23.1 Bn in 2032, growing at a CAGR of 8.9% during the forecast period 2025-2032. Clinical developments are driving the need for vascular stents. In addition, shifting treatment protocols, and expanding procedural volumes across both mature and emerging healthcare systems. Stents have evolved from commoditized devices into precision-engineered solutions made for complex anatomies and comorbid patient groups. The surge of value-based healthcare and increasing demand for minimally invasive interventions is further encouraging manufacturers to invest in novel stent technologies. These often range from polymer-free drug-eluting platforms to bioresorbable scaffolds.
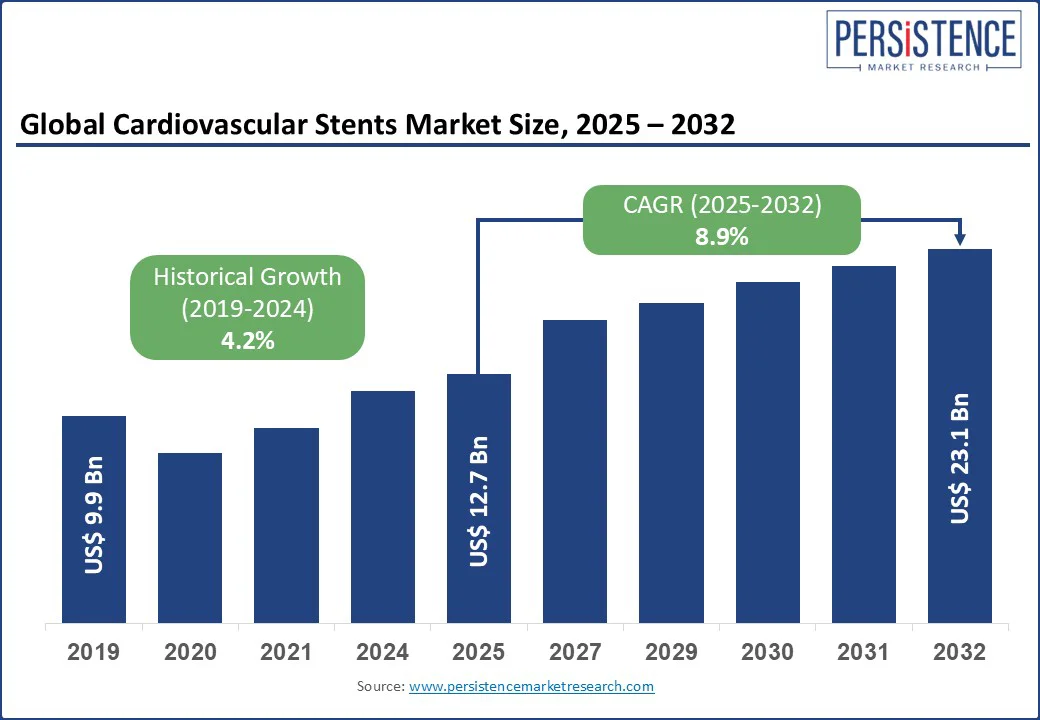
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Cardiovascular Stents Market Size (2025E) |
US$ 12.7 Bn |
|
Market Value Forecast (2032F) |
US$ 23.1 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
8.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.2% |
The increasing prevalence of cardiovascular diseases (CVDs), specifically Coronary Artery Disease (CAD), is pushing the cardiovascular stents market growth. This is because stenting remains one of the most common interventional treatments for obstructed arteries. According to the Global Burden of Disease Study published in The Lancet in 2023, ischemic heart disease remains the leading cause of death worldwide, accounting for over 9 Mn deaths annually. This surge is evident in low- and middle-income countries, where lifestyle transitions are spurring the incidence of atherosclerosis and related complications.
Healthcare institutions are hence striving to extend access to Percutaneous Coronary Interventions (PCIs), which inherently boosts stent use. The age-related nature of CVDs also plays an important role in market growth. In high-income countries such as the U.S., over 40% of individuals aged 60 and above have some form of heart disease, with many requiring stent implantations during angioplasty, found the National Institutes of Health (NIH). In emerging economies, the burden of untreated or late-diagnosed heart disease is pushing governments to extend interventional cardiology programs, thereby fueling demand.
Bleeding and infection at the insertion site, along with allergic reactions to contrast dyes and stent materials, remain key concerns hampering the wide adoption of cardiovascular stents. It is evident in patients with high bleeding risk or comorbidities. According to a 2023 analysis published in JACC: Cardiovascular Interventions, approximately 6 to 8% of patients undergoing PCI experience some form of access-site complication such as hematoma or bleeding. It leads to prolonged hospitalization and increases the risk of mortality.
Such complications are more prevalent among elderly patients, those on anticoagulant therapy, and individuals with peripheral artery disease. This makes physicians cautious about recommending stent procedures in such populations. Allergic reactions to iodinated contrast agents used during angiography further complicate the scenario. Although rare, these reactions often range from mild urticaria to severe anaphylaxis. The risk is concerning in patients with pre-existing renal impairment, where contrast-induced nephropathy tends to lead to acute kidney injury.
Ongoing research into stent design, material development, and surface engineering is creating new opportunities by targeting previously underserved clinical segments. An important development is the emergence of ultra-thin strut Drug-Eluting Stents (DES), which have demonstrated reduced vessel trauma and fast endothelialization. Such design improvements are enabling safe stenting in small vessels and complex anatomies, further broadening indications and procedural volumes.
Researchers are also exploring magnesium-based bioresorbable stents that naturally dissolve after arterial healing, reducing long-term risks such as late stent thrombosis. The MAGNITUDE trial, published in 2024, analyzed a new generation of resorbable magnesium scaffolds and reported encouraging results in terms of vascular remodeling and minimal inflammatory response. Such devices are promising for young patients or those requiring future reinterventions, creating new commercial avenues in structurally healthy yet high-risk populations.
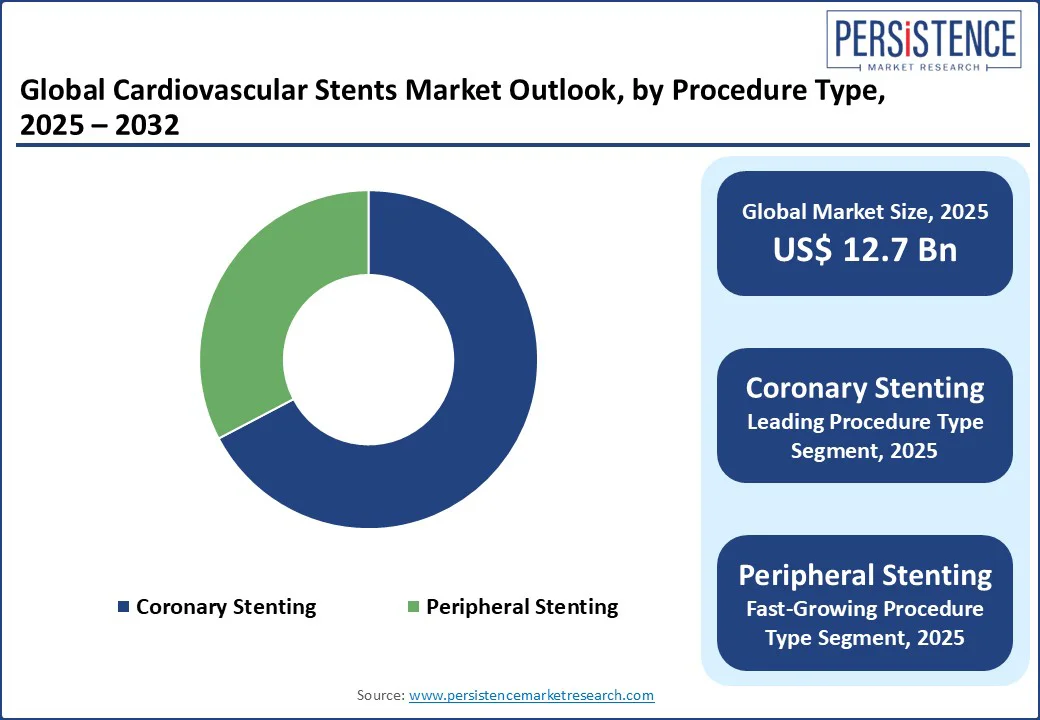
Based on procedure type, the market is bifurcated into coronary stenting and peripheral stenting. Out of these, coronary stenting is predicted to hold about 67.3% of share in 2025 owing to its minimally invasive nature, quick symptom relief, and proven efficacy in reducing adverse cardiovascular events. The procedure enables immediate revascularization of narrowed coronary arteries without the requirement for open-heart surgery. This makes it ideal for elderly patients or those with multiple comorbidities who are poor surgical candidates.
Peripheral stenting is gaining momentum, backed by the surging prevalence of Peripheral Artery Disease (PAD), specifically among aging populations and diabetic patients. In 2023, the Global PAD Burden Report estimated that over 230 Mn people worldwide are living with PAD, with a notable number developing Critical Limb Ischemia (CLI). Stenting has emerged as a preferred alternative to open vascular surgery for revascularizing peripheral arteries. This is attributed to its reduced recovery time, procedural simplicity, and cost-effectiveness.
By stent type, the market is divided into Drug Eluting Stents (DES), bioresorbable stents, bare metal stents, and others. Among these, DES will likely account for nearly 65.2% of the cardiovascular stents market share in 2025 due to their ability to lower restenosis and repeat revascularization in high-risk and complex lesions. Coronary DES release antiproliferative drugs that help prevent neointimal hyperplasia, a primary cause of in-stent restenosis. This pharmacological coating has led to a dramatic reduction in Target Lesion Revascularization (TLR) rates.
Bioresorbable stents are exhibiting steady growth owing to their potential to eliminate long-term complications associated with permanent metallic implants, including late stent thrombosis. These stents provide temporary mechanical support to the vessel during healing and gradually dissolve, leaving behind a restored and unobstructed artery. This feature is appealing to young patients or those expected to require repeat interventions, where preserving vascular flexibility is important.
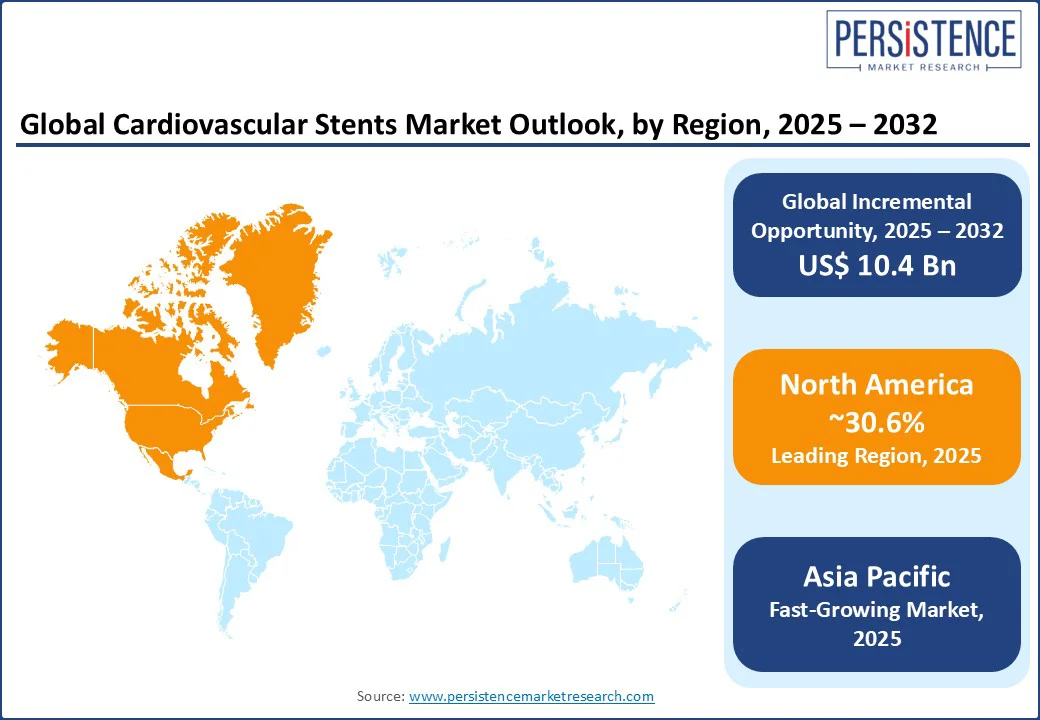
North America is predicted to account for approximately 30.6% of share in 2025 due to high disease prevalence and increasing adoption of novel stent technologies. The U.S. cardiovascular stents market continues to dominate with a large aging population and rising incidence of CAD. According to the Centers for Disease Control and Prevention (CDC), in 2022 alone, CAD killed nearly 371,506 individuals in the U.S. In addition, about 1 in 20 adults aged 20 years and older have CAD.
The demographic directly feeds into the demand for both drug-eluting and bare-metal stents. The market is further shifting toward next-generation DES with thin struts and biodegradable polymers. This is because hospitals are mainly prioritizing long-term patient outcomes and reduced restenosis rates. Manufacturers in the cardiac stent market are improving product portfolios for quick deployment and compatibility with catheter-based interventions as Medicare reimbursement reforms are encouraging minimally invasive procedures and same-day discharge.
The market in Europe is experiencing a transitional phase owing to evolving regulatory frameworks, increasing clinical scrutiny, and regional disparities in technology adoption. The implementation of the EU Medical Device Regulation (MDR) in 2021 has drastically delayed the re-certification and market entry of several cardiovascular stents. It has prompted multinational companies to prioritize the U.S. and Asia Pacific for quick regulatory clearance.
As per the European Commission, as of early 2024, over 30% of previously CE-marked cardiovascular devices had not yet been re-certified under the European Union Medical Device Regulation. It has led to constrained supply in a few markets. Germany leads in the adoption of intravascular imaging technologies such as IVUS and OCT alongside DES. It is attributed to its unique reimbursement policies and high volume of PCI-capable centers. The U.K. has also introduced its regulatory framework via the Medicines and Healthcare products Regulatory Agency (MHRA), leading to parallel approval processes that slightly slow down access to the latest DES technologies.
Asia Pacific is being spurred by surging PCIs across populous countries, including China and India. China has witnessed a steady growth in stent implantations, with more than 1.7 Mn PCI procedures conducted in 2023 alone, as per the National Cardiovascular Center of China. This surge is associated with the country’s Volume-Based Procurement (VBP) policy, which has lowered stent prices since its 2021 rollout. It has pressured multinational brands while simultaneously boosting the uptake of domestically manufactured stents.
India’s market, though smaller in absolute volume than China is similarly dynamic. The country capped stent prices in 2017, and as of 2024, the price ceiling remains in place, prompting global players to reconsider their pricing models or limit portfolio availability. Domestic companies such as Sahajanand Medical Technologies and Meril Life Sciences have thrived, utilizing cost efficiency and clinical trial data to gain share in both domestic and international markets.
The cardiovascular stents market is characterized by well-established companies and disruptive innovators, with an emphasis on technological differentiation, partnerships, and geographic expansion. Key players are dominating through diversified product portfolios and global distribution networks. Their share, however, is being challenged by specialized firms that focus on niche technologies and regional strengths. DES remain the leading point of development, with next-generation products incorporating bioresorbable polymers, ultra-thin strut designs, and controlled drug-release profiles. Strategic acquisitions and licensing deals are also pushing the market.
The cardiovascular stents market is projected to reach US$ 12.7 Bn in 2025.
Increasing adoption of minimally invasive procedures and rising global prevalence of coronary artery disease are the key market drivers.
The cardiovascular stents market is poised to witness a CAGR of 8.9% from 2025 to 2032.
Regulatory support for fast-tracked approval of novel stent technologies and expansion of peripheral stenting are the key market opportunities.
Biotronik SE & Co. KG, Cardinal Health, Inc., and Cook Medical are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Procedure Type
By Stent Type
By Disease Indication
By End Use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author