ID: PMRREP19135| 202 Pages | 10 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

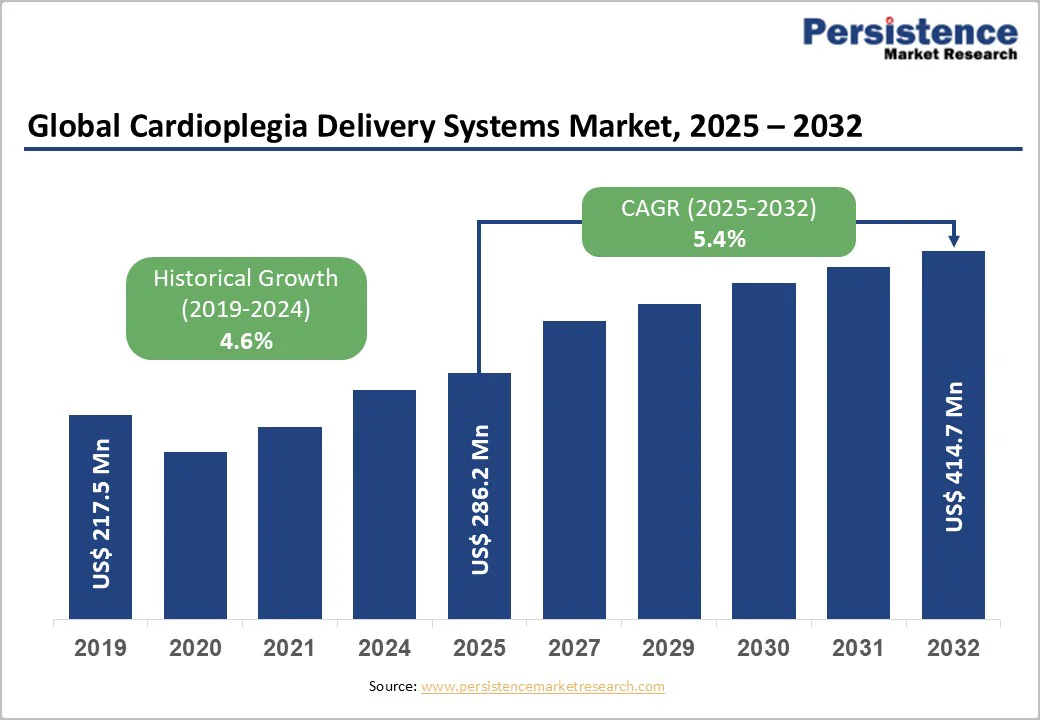
The global cardioplegia delivery systems market size is valued at US$ 286.2 million in 2025 and is projected to reach US$ 414.7 million by 2032, growing at a CAGR of 5.4% between 2025 and 2032.
A cardioplegia delivery system is a specialized medical device used during open-heart surgeries to provide precise myocardial protection. It enables controlled administration of cardioplegia solutions, inducing rapid and safe cardiac arrest while maintaining a stable surgical field. By protecting myocardial cells from ischemic injury, the device supports recovery of cardiac function post-surgery.
| Key Insights | Details |
|---|---|
|
Cardioplegia Delivery Systems Market Size (2025E) |
US$ 286.2 Million |
|
Market Value Forecast (2032F) |
US$ 414.7 Million |
|
Projected Growth (CAGR 2025 to 2032) |
5.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.6% |
The global cardioplegia delivery systems market is driven by the rising prevalence of cardiovascular disease (CVD), increasing cardiac surgery volumes, and continuous advancements in myocardial protection techniques. CVD remains the leading cause of death worldwide, responsible for nearly 18 million fatalities annually, with over 80% of mortality occurring in low- and middle-income countries (LMICs). Globally, more than one million cardiac surgical procedures are performed each year, including coronary artery bypass grafting (CABG), valvular surgery, and congenital heart surgery.
In high-income countries (HICs), the average annual cardiac surgical volume is approximately 123.2 procedures per 100,000 population, including 36.7 CABG, 30.8 valvular, and 7.9 congenital surgeries. For upper-middle-, lower-middle-, and low-income countries, adjusted surgical targets are 86.1, 55.1, and 40.2 procedures per 100,000 population, respectively.
Coupled with innovations in cardioplegia techniques that enhance myocardial protection, procedural efficiency, and patient safety, these trends collectively propel adoption of advanced cardioplegia delivery systems across global markets.
The global cardioplegia delivery systems market faces restraints from infrastructure limitations and workforce capacity, particularly in low- and middle-income countries (LMICs). Many healthcare facilities require upgraded surgical infrastructure, trained perfusionists, and standardized protocols to ensure safe and effective myocardial protection. Bridging these gaps demands significant investment and coordinated training efforts, which can slow adoption of advanced cardioplegia technologies.
Additionally, clinical complications related to improper cardioplegia administration further constrain market growth. Inadequate cardioplegic protection can result from incorrect solution preparation, poor administration technique, failure to re-dose short-acting formulations, or patient-specific factors such as severe coronary artery disease (CAD).
Potential consequences include ischemia-reperfusion injury, electrolyte imbalances, cooling-related complications, cardioplegia toxicity, infection risk, and accidental systemic injection. Even when procedures are executed correctly, insufficient monitoring or deviation from standards of care can escalate minor errors into serious, sometimes fatal, outcomes. These clinical risks, combined with infrastructure and workforce challenges, limit widespread adoption of cardioplegia delivery systems, particularly in resource-constrained settings.
The increasing adoption of Del Nido cardioplegia in adult cardiac surgeries presents a significant opportunity for manufacturers to develop specialized, multi-dose, high-precision cardioplegia delivery systems.
Del Nido cardioplegia is increasingly utilized for procedures with prolonged aortic cross-clamp (AXC) times, which necessitate extended ischemic protection and careful dosing. Recent research studies highlights that between June 12, 2014, and December 31, 2022, 5,094 adult cardiac surgeries were performed at Flinders Medical Centre and Flinders Private Hospital, of which 845 cases involved AXC times greater than 90 minutes.
Data from the Flinders Cardiac Surgery Registry and the Australian and New Zealand Collaborative Perfusion Registry demonstrated comparable outcomes between Del Nido and hyperkalaemic cardioplegia, highlighting opportunities to optimize delivery systems for complex, long-duration procedures.
Ongoing clinical trials and research further expand market potential. For example, studies in China (November 2024–October 2025) compare novel solutions with histidine–tryptophan–ketoglutarate (HTK) cardioplegia, while the DESTINY trial in the UK evaluates Del Nido versus St. Thomas’ blood cardioplegia in pediatric congenital heart surgery. Additional research exploring adjuncts underscores the need for advanced, adaptable delivery platforms globally.
Automated delivery systems are expected to capture a leading 35.3% share of the global cardioplegia delivery systems market by 2025. These systems offer precise control over flow rates, temperature, and dosing, reducing the risk of human error during complex cardiac procedures. By standardizing delivery, automated systems enhance myocardial protection, improve surgical efficiency, and support consistent outcomes across diverse patient populations. Growing adoption of advanced perfusion technologies in high-volume cardiac centers and increasing demand for minimally invasive surgeries further drive market preference for automated cardioplegia delivery platforms globally.
Crystalloid cardioplegia is projected to dominate the global cardioplegia delivery systems market in 2025, capturing nearly 44.7% of the total share. Its wide adoption stems from simplicity in preparation, predictable myocardial protection, and proven clinical safety. Crystalloid solutions are preferred in both adult and pediatric cardiac surgeries due to cost-effectiveness, ease of administration, and compatibility with automated delivery systems. Increasing cardiac surgery volumes, emphasis on standardized myocardial protection protocols, and extensive clinical familiarity contribute to crystalloid cardioplegia’s leading market position across regions, including North America, Europe, and the Asia Pacific.
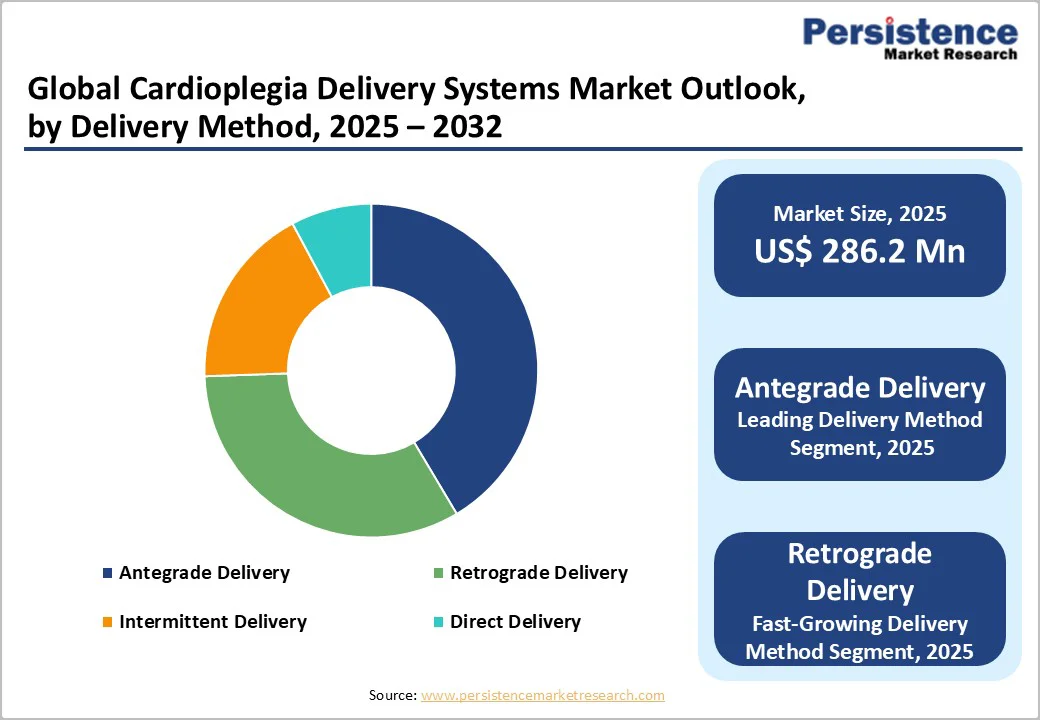
The antegrade delivery method is expected to account for a leading 38.4% share of the global cardioplegia delivery systems market by 2025. Antegrade cardioplegia involves infusion directly into the coronary arteries, ensuring rapid and uniform myocardial protection. It is widely preferred for standard coronary artery bypass grafting (CABG) and complex valvular surgeries. Advantages include predictable distribution, reduced ischemic injury, and compatibility with both crystalloid and blood-based solutions. Growing cardiac surgery volumes, rising adoption of automated perfusion technologies, and established clinical guidelines further reinforce antegrade delivery’s dominance in the cardioplegia market globally.
CABG is projected to dominate the global cardioplegia delivery systems market in 2025, capturing nearly 31.8% of the total share. CABG is performed to bypass blocked coronary arteries, restoring oxygen-rich blood to the heart muscle and preventing heart attacks. With around 200,000 procedures annually in the U.S., CABG remains the most common cardiac surgery worldwide (Society for Cardiovascular Angiography & Interventions, SCAI 2025). The critical need for effective myocardial protection during these high-risk, open-heart procedures drives the demand for advanced cardioplegia delivery systems, including automated and precision-controlled platforms, ensuring improved surgical outcomes globally.
Hospitals are expected to account for a leading 47.6% share of the global cardioplegia delivery systems market by 2025. Hospitals provide the necessary infrastructure, skilled perfusionists, and surgical teams required for advanced cardiac procedures. The high volume of cardiac surgeries performed in hospitals, combined with adoption of automated delivery systems and adherence to standardized myocardial protection protocols, drives demand. Additionally, hospitals’ focus on patient safety, efficiency, and integration with existing perfusion technologies reinforces their position as the primary end-user of cardioplegia delivery systems worldwide.
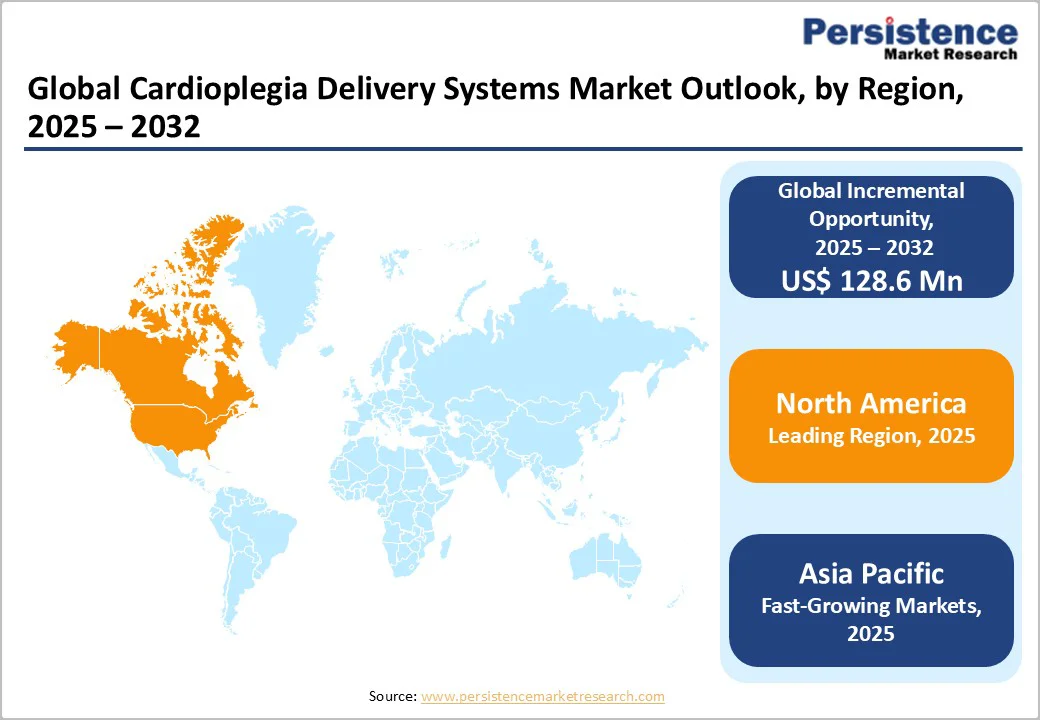
By 2025, North America is projected to capture approximately 34.4% of the global cardioplegia delivery systems market, driven by robust clinical infrastructure and technological advancements in cardiac surgery.
The Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) is the most comprehensive registry for adult cardiac procedures in the United States, tracking over 8.3 million surgeries across more than 1,000 institutions and covering more than 97% of adult cardiac surgeries (ACSD, 2023). The ACSD is part of the broader STS National Database, which provides a national benchmark for cardiothoracic surgery, with data on nearly 10 million procedures from over 4,300 surgeons.
Additionally, in August 2025, Spectrum Medical received 510(k) approval for the Quantum Micro-Cardioplegia Delivery System, enabling precise myocardial protection and seamless integration with existing perfusion platforms. These advancements in clinical databases and innovative delivery systems are expected to strengthen procedural efficiency, enhance patient safety, and drive adoption of cardioplegia technologies across North America.
By 2025, Europe is projected to hold nearly 28.6% of the global cardioplegia delivery systems market, driven by strong clinical standards, guideline adoption, and technological advancements in cardiac surgery.
In 2024, the European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Cardiothoracic Anaesthesiology and Intensive Care (EACTAIC), and the European Board of Cardiovascular Perfusion (EBCP) established a joint task force to update cardiopulmonary bypass (CPB) guidelines for adult cardiac surgery.
The guidelines were rigorously reviewed by an external panel of experts and ratified by the leadership of all three organizations, with publication in leading journals. These guidelines emphasize standardized CPB protocols, patient safety, and myocardial protection, fostering consistent clinical practice across Europe.
The structured recommendations, coupled with ongoing technological innovation in cardioplegia delivery systems, are expected to enhance procedural efficiency, optimize surgical outcomes, and drive market adoption throughout the region.
Asia Pacific cardioplegia delivery systems market is projected to grow at a CAGR of 6.8% through 2032, driven by the rising burden of cardiovascular disease (CVD) and increasing adoption of advanced cardiac surgical technologies.
In 2019, Asia accounted for nearly 60% of the 18.6 million CVD deaths recorded globally, with crude cardiovascular mortality expected to rise by 91.2% from 2025 to 2050, reaching 24.1 million deaths (World Heart Federation, 2025). East Asia, South Asia, and South-East Asia are projected to see the largest increases in mortality, at 147.4%, 85.3%, and 81.6%, respectively.
Key risk factors include high blood pressure, metabolic conditions, unhealthy diets, and air pollution, which alone contributes to 28% of CVD-related deaths in South-East Asia. The escalating disease burden, coupled with technological advancements in cardioplegia delivery systems, is driving adoption in hospitals, improving myocardial protection, surgical efficiency, and patient outcomes across the region.
The cardioplegia delivery systems market is highly competitive, driven by rapid technological innovations, and increasing adoption of advanced myocardial protection solutions. Key players focus on product differentiation through efficiency, safety, and integration with existing perfusion systems, while regional expansions and clinical validations further intensify market competition.
The global cardioplegia delivery systems market is projected to be valued at US$ 286.2 Million in 2025.
Rising cardiovascular disease prevalence, increasing cardiac surgeries, advancements in myocardial protection technologies, stringent clinical guidelines, and growing focus on surgical efficiency and patient safety are the primary factors driving market growth globally.
The global cardioplegia systems market is poised to witness a CAGR of 5.4% between 2025 and 2032.
Expansion in emerging regions, development of minimally invasive and automated cardioplegia delivery systems, integration with perfusion technologies, and adoption of ultra-low volume solutions present significant growth opportunities for the market.
Major players in the global are Medtronic, LivaNova, Inc., NIPRO, Weigao Group, Terumo Cardiovascular Systems Corporation, and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn, Volume (Units) |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Cardioplegia Solution
By Delivery Method
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author