ID: PMRREP35586| 193 Pages | 3 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

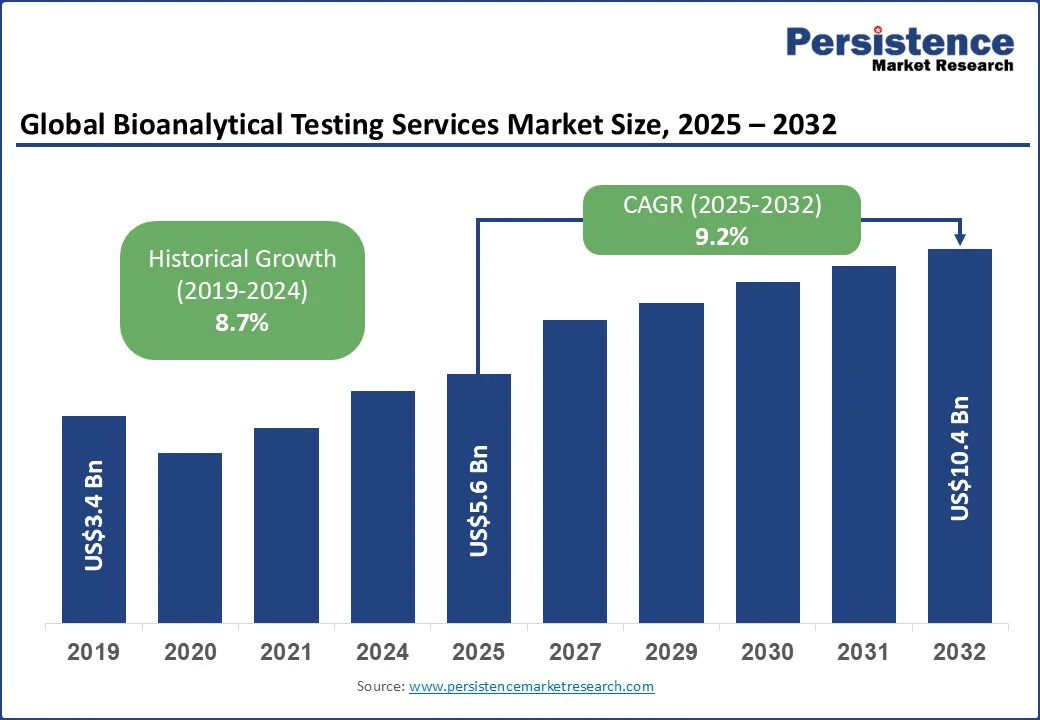
The global bioanalytical testing services market size is projected to rise from US$5.6 Bn in 2025 to US$10.4 Bn by 2032. It is anticipated to witness a CAGR of 9.2% during the forecast period from 2025 to 2032.
The bioanalytical testing services market growth is being fueled by the development of complex therapeutic modalities and the implementation of strict guidelines on drug development.
Leading players are embracing integrated strategies to maintain a competitive advantage. They are investing in hybrid assay platforms that combine LC-MS, ligand-binding, and molecular techniques under a single Quality Management System (QMS). This enables them to handle complex biologics, gene therapies, and Advanced Therapy Medicinal Products (ATMPs) in one streamlined workflow.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Bioanalytical Testing Services Market Size (2025E) |
US$5.6 Bn |
|
Market Value Forecast (2032F) |
US$10.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
9.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
8.7% |
The development of biologics and gene therapies is propelling the demand for bioanalytical testing as these modalities are inherently complex. These require specialized assays to measure pharmacokinetics, immunogenicity, and biodistribution.
Biologics such as monoclonal antibodies, bispecifics, and fusion proteins demand hybrid platforms combining ligand-binding assays and LC-MS to capture total and free drug concentrations. Gene therapies increase complexity as regulators require constant monitoring of vector shedding, biodistribution, and immune responses.
High-sensitivity qPCR and digital PCR methods are essential to detect low-copy viral genomes in diverse matrices, while neutralizing antibody assessments ensure patient safety. The rise of personalized medicine also contributes to this trend. Biologics and gene therapies are often developed for small patient populations or rare diseases. These necessitate highly precise and validated bioanalytical methods that can accommodate limited sample volumes.
The regulatory framework for bioanalytical laboratories is becoming complex, which is hampering the efficiency of testing services. Compliance with ICH M10, GLP, GCP, and local country-specific requirements demands extensive documentation, validated methods, and routine audits. Several small-scale labs struggle to keep up with these overlapping standards, which limits their ability to take on high-complexity or multi-site studies. Licensing and inspection protocols also impose operational bottlenecks.
Bioanalytical labs handling ATMPs or viral vectors must meet BSL-2 or BSL-3 containment requirements and comply with additional reporting for vector shedding, biodistribution, and immunogenicity. This layer of technical and regulatory compliance often compels sponsors to rely on large and fully accredited CROs. It further limits competition and slows down access for small innovators. Frequent guidance changes, including the EMA’s updates on ATMP assay expectations, also force labs to revalidate methods or invest in new instrumentation to stay compliant.
The establishment of specialized outsourcing firms is expanding avenues for bioanalytical testing services. These provide pharma and biotech companies with access to novel technologies without the requirement for in-house capabilities.
These firms deliver hybrid assay platforms, high-resolution mass spectrometry, and automated sample preparation systems that enable sponsors to accelerate clinical trials while maintaining regulatory compliance. In 2025, Frontage Laboratories, Inc., for example, broadened its U.S. facilities, improving its ability to provide integrated bioanalytical services.
Outsourcing firms also facilitate global trial operations by bridging regional regulatory requirements. Key players, such as Syneos Health and Eurofins, have developed multi-site networks that harmonize bioanalytical workflows across the U.S., Europe, and Asia Pacific.
These help ensure consistent and audit-ready data for regulatory submissions. Small and mid-size biotech firms benefit from the ability to outsource high-complexity assays without investing in expensive instrumentation or hiring specialized staff.
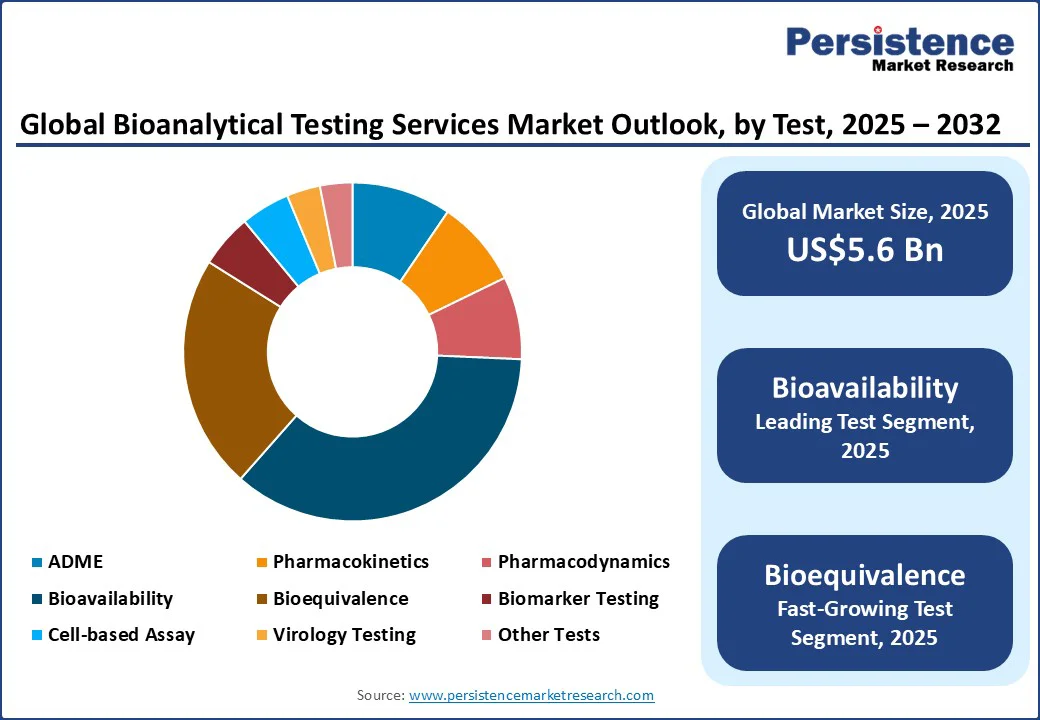
Based on the test, the market is divided into ADME, pharmacokinetics (PK), pharmacodynamics (PD), bioavailability, bioequivalence, biomarker testing, cell-based assay, virology testing, and other tests. Among these, bioavailability testing is predicted to hold nearly 35.8% of the market share in 2025 as it provides direct insight into how a drug behaves in the human body, which is important for both regulatory approval and therapeutic effectiveness.
These tests help measure the actual rate and extent at which the active pharmaceutical ingredient enters systemic circulation. This gives sponsors confidence that the formulation will deliver the intended clinical effect.
Bioequivalence testing is experiencing steady growth as it ensures that generic or alternative formulations deliver the same therapeutic effect as their reference drugs without the requirement for extensive clinical trials. Regulators such as the FDA and EMA often rely on bioequivalence data to grant marketing approval for generics.
This makes the testing a quick and cost-effective pathway to market. In addition, the surge in complex generics and modified-release formulations is fueling demand.
By end-user, the market is segregated into pharma and biopharma companies, CDMO, CRO, and others. Out of these, pharma and biopharma companies are expected to account for approximately 54.8% share in 2025 as their business model revolves around developing safe and effective therapeutics.
Precise measurements of drug exposure, metabolism, and immunogenicity are also important. These companies rely on bioanalytical data to make informed decisions during preclinical and clinical development. They are also focused on complex modalities, which require highly specialized assays.
Contract Research Organizations (CROs) are key end-users as they act as the operational backbone for pharma and biopharma companies. These help deliver specialized testing without the sponsor needing to maintain extensive in-house capabilities.
CROs handle multiple modalities, often running pharmacokinetics, immunogenicity, and biomarker assays across global clinical trials. They also drive demand by meeting stringent regulatory requirements for clients across different regions.
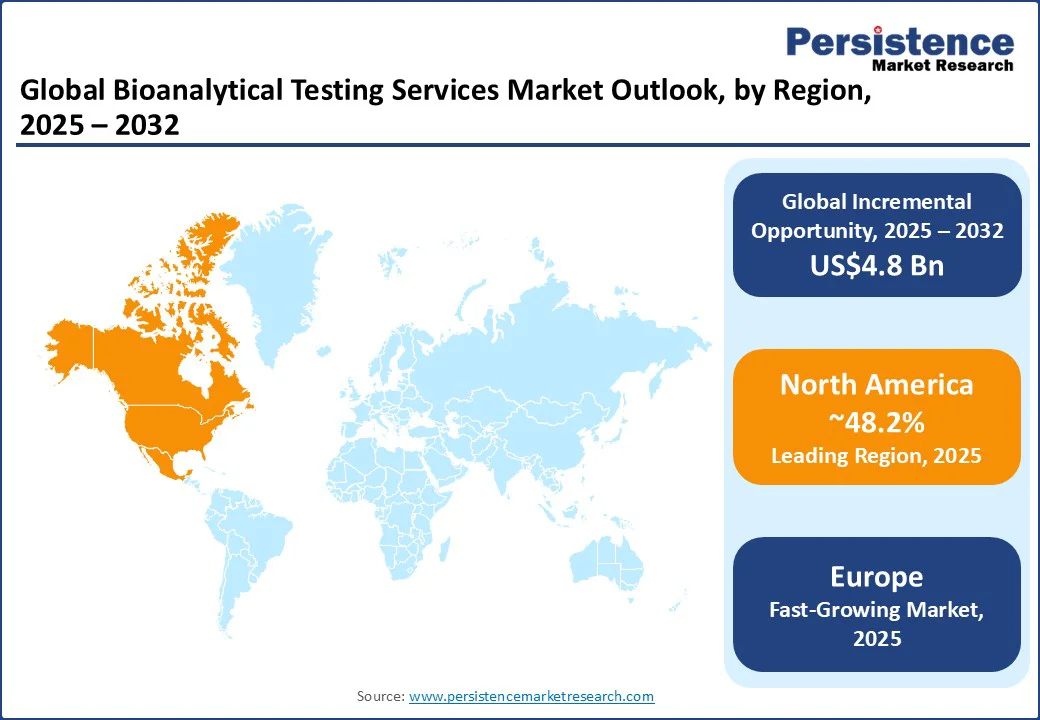
In 2025, North America will likely account for around 48.2% of the share as the market is settling into a new normal influenced by harmonized rules and complex modalities. The U.S. Food and Drug Administration’s (FDA) final ICH M10 guidance, issued in June 2024, locked in common expectations for validating chromatographic and ligand-binding assays. This shift is pushing sponsors and CROs to standardize method plans and documentation across sites, which trims re-validation cycles and eases audits. Health Canada points to the same ICH M10 text and Q&As.
Gene and cell therapy programs are boosting growth in the U.S. bioanalytical testing services market. Study packages now seek integrated biodistribution, vector-shedding, and immunogenicity workflows. The FDA updated its cellular and gene therapy guidance hub in June 2025, including finalized thinking on how to design and analyze shedding studies. Recent community pieces laid out validated qPCR and digital PCR routes for AAV biodistribution and shedding, which are now routine tasks in first-in-human trials.
Europe’s market is now operating on a common rulebook, and that matters in day-to-day lab work. ICH M10 has been in force across the EU since January 2023, replacing the old EMA bioanalytical guideline. It has unified expectations for validation and study sample analysis. The U.K. has also implemented ICH M10 via the Medicines and Healthcare products Regulatory Agency (MHRA). Sponsors are writing one method plan and using it on both sides of the channel, rather than retooling for local quirks.
Novel therapies are setting the pace for new assay types in Europe. European Medicines Agency (EMA) and the Commission have upgraded the ATMP playbook, with a 2025 clinical-stage guideline and CAT guidance on AAVs, biodistribution, and environmental risk. Service providers are also developing hybrid labs that host LC-MS, ligand-binding platforms, and molecular (qPCR/dPCR) workflows under the same QMS. Recent facility investments show that providers are chasing integrated and inspection-ready stacks rather than one-off instrument buys.
Asia Pacific is considered a patchwork of fast-maturing hubs rather than a single market. China and Japan push regulator-led technical depth, while India and Korea bolster volume and cost-effective trial execution. Singapore acts as a specialized hub for cell-and-gene analytics as well as regional coordination. Two operational realities shape procurement across Asia Pacific.
ATMPs and complex biologics augment demand for hybrid labs that run LC-MS, ligand-binding, and molecular assays under one QMS. In addition, decentralized sampling, micro-volume stability validation, and reliable cold-chain couriers are now explicit line items in bids. Recent investments and cross-border deals from regional CROs illustrate providers racing to meet those checks.
The global bioanalytical testing services market is dominated by a few global CRO giants that cover every stage of development. Key players often bundle regulated bioanalysis with preclinical and clinical trial support. Their position allows them to invest in high-resolution mass spectrometry, hybrid assay platforms, and end-to-end data systems.
This reach makes them the default choice for multinational trials where inspection readiness and geographic spread are non-negotiable. Mid-tier players focus on delivering agility that large-scale CROs struggle to replicate. Some concentrate on difficult modalities such as immunogenicity testing for biologics, while others are early adopters of micro-volume workflows.
The bioanalytical testing services market is projected to reach US$5.6 Bn in 2025.
The surging focus on rare-disease therapeutics and rising demand for biologics are the key market drivers.
The bioanalytical testing services market is expected to grow at a CAGR of 9.2% from 2025 to 2032.
The expansion of outsourcing partnerships and the adoption of decentralized trial sampling are key market opportunities.
Thermo Fisher Scientific Inc., Charles River Laboratories International, and ICON Plc are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Molecule
By Test
By Workflow
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author