- Executive Summary
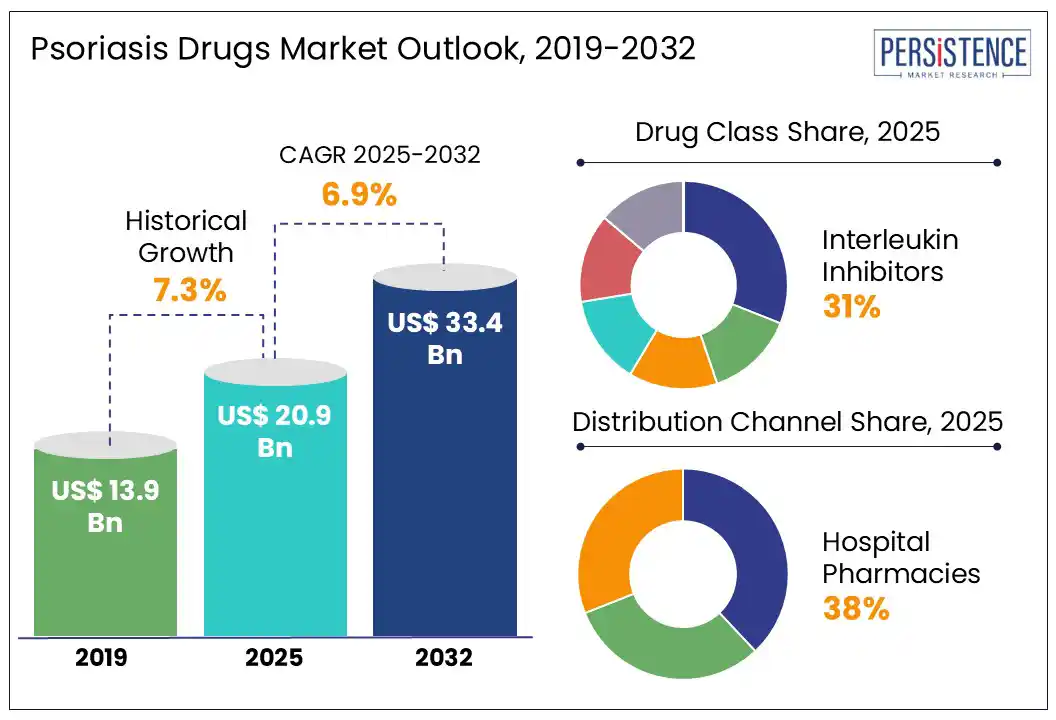
- Global Psoriasis Drugs Market Snapshot, 2025 and 2032
- Market Opportunity Assessment, 2025 - 2032, US$ Mn
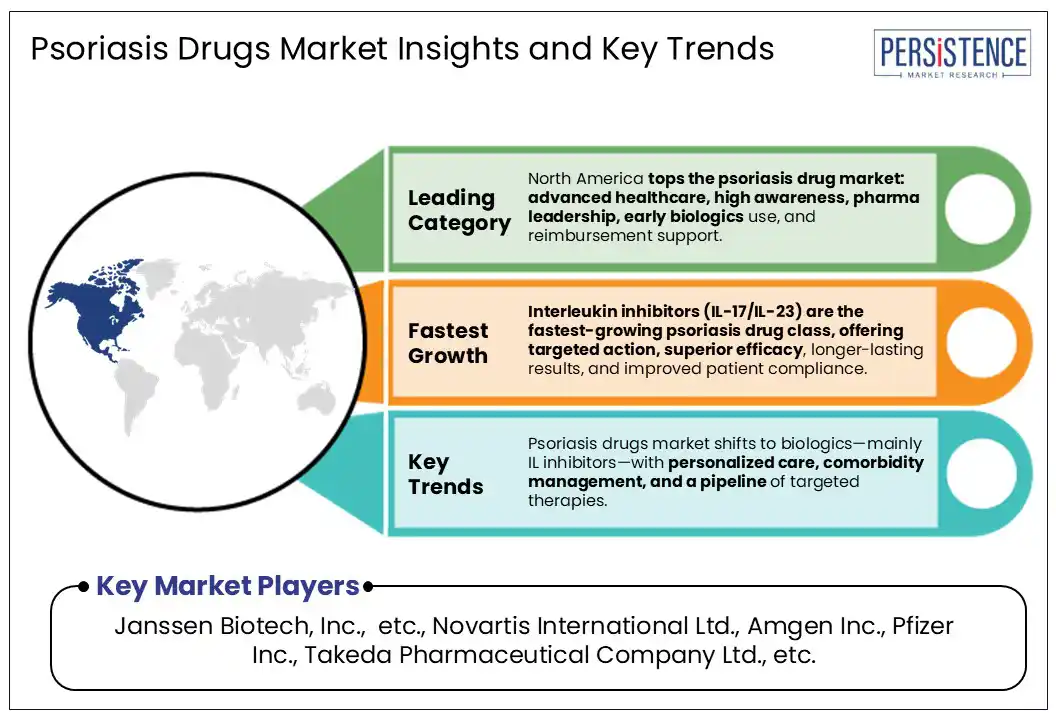
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments and Key Market Events
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Key Trends
- Macro-Economic Factors
- Global Sectorial Outlook
- Global GDP Growth Outlook
- COVID-19 Impact Analysis
- Forecast Factors - Relevance and Impact
- Value Added Insights
- Drug Class Adoption Analysis
- Regulatory Landscape
- Pipeline Assessment
- Disease Epidemiology
- Key Deals and Mergers
- PESTLE Analysis
- Value Chain Analysis
- Porter’s Five Force Analysis
- Global Psoriasis Drugs Market Outlook:
- Key Highlights
- Market Size (US$ Mn) and Y-o-Y Growth
- Absolute $ Opportunity
- Market Size (US$ Mn) Analysis and Forecast
- Historical Market Size (US$ Mn) Analysis, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, 2025-2032
- Global Psoriasis Drugs Market Outlook: Drug Class
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Drug Class, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Attractiveness Analysis: Drug Class
- Global Psoriasis Drugs Market Outlook: Mode of Administration
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Mode of Administration, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Attractiveness Analysis: Mode of Administration
- Global Psoriasis Drugs Market Outlook: Indication
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Indication, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Attractiveness Analysis: Indication
- Global Psoriasis Drugs Market Outlook: Distribution Channel
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Distribution Channel, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis: Distribution Channel
- Key Highlights
- Global Psoriasis Drugs Market Outlook: Region
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Region, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, By Region, 2025-2032
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast By Country, 2025-2032
- U.S.
- Canada
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- Europe Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast, By Country, 2025-2032
- Germany
- France
- U.K.
- Italy
- Spain
- Russia
- Türkiye
- Rest of Europe
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- East Asia Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast, By Country, 2025-2032
- China
- Japan
- South Korea
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- South Asia & Oceania Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast, By Country, 2025-2032
- India
- Southeast Asia
- ANZ
- Rest of South Asia & Oceania
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- Latin America Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast, By Country, 2025-2032
- Brazil
- Mexico
- Rest of Latin America
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- Middle East & Africa Psoriasis Drugs Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019-2024
- By Country
- By Drug Class
- By Mode of Administration
- By Indication
- By Distribution Channel
- Market Size (US$ Mn) Analysis and Forecast, By Country, 2025-2032
- GCC Countries
- Egypt
- South Africa
- Northern Africa
- Rest of Middle East & Africa
- Market Size (US$ Mn) Analysis and Forecast, By Drug Class, 2025-2032
- Interleukin Inhibitors
- Corticosteroids
- Anti-inflammatory
- Glucocorticoids
- Tnf alfa Inhibitor
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Mode of Administration, 2025-2032
- Topical
- Oral
- Injectable
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Indication, 2025-2032
- Guttate Psoriasis
- Inverse Psoriasis
- Pustular Psoriasis
- Erythrodermic Psoriasis
- Plaque Psoriasis
- Others
- Market Size (US$ Mn) Analysis and Forecast, By Distribution Channel, 2025-2032
- Hospital Pharmacies
- Retail Pharmacies
- e-Commerce
- Market Attractiveness Analysis
- Competition Landscape
- Market Share Analysis, 2024
- Market Structure
- Competition Intensity Mapping By Market
- Competition Dashboard
- Company Profiles (Details - Overview, Financials, Strategy, Recent Developments)
- Janssen Biotech, Inc.
- Overview
- Segments and Drug Class
- Key Financials
- Market Developments
- Market Strategy
- Novartis International Ltd.
- Amgen Inc.
- Pfizer Inc.
- Takeda Pharmaceutical Company Ltd.
- Merck & Co, Inc.
- AbbVie Inc.
- Eli Lilly and Company
- Boehringer Ingelheim GmbH
- Sun Pharmaceutical Industries Ltd.
- Johnson & Johnson
- LEO Pharma
- UCB
- AstraZeneca
- Celgene Corporation
- Arcutis Biotherapeutics, Inc.
- Others
- Janssen Biotech, Inc.
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment