ID: PMRREP3403| 200 Pages | 7 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

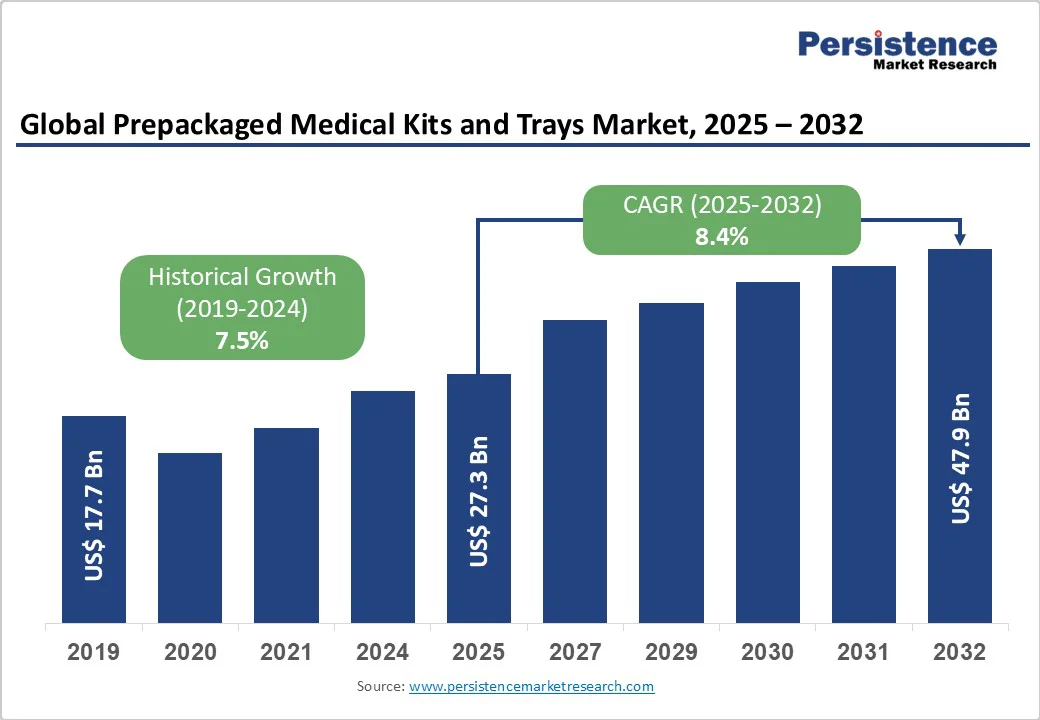
The global prepackaged medical kits & trays market size is likely to reach US$27.3 billion in 2025 and is projected to reach US$47.9 billion by 2032, growing at a CAGR of 8.4% between 2025 and 2032. The prepackaged medical kits and trays market is gaining strong traction as healthcare systems worldwide prioritize efficiency, safety, and cost control.
These ready-to-use, procedure-specific kits streamline surgical preparation, reduce operating room turnover times, and minimize risks of contamination, making them indispensable in hospitals, ambulatory surgical centers, and outpatient clinics.
| Key Insights | Details |
|---|---|
|
Global Prepackaged Medical Kits & Trays Market Size (2025E) |
US$ 27.3 Billion |
|
Market Value Forecast (2032F) |
US$ 47.9 Billion |
|
Projected Growth (CAGR 2025 to 2032) |
8.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.5% |
Increasing number of surgical procedures worldwide, especially minimally invasive ones, and a shift toward outpatient and ambulatory settings is driving the global prepackaged medical kits & trays market. In the U.S., the volume of ASC surgical procedures per fee-for-service (FFS) beneficiary rose by 5.7% in 2023, following an annual average growth of 0.6% between 2018 and 2022.
Studies show many general surgical operations (e.g. mastectomy, thyroid lobectomy, minimally invasive hernia repair) have seen significant increases in outpatient performance over the past decade. These trends raise demand for ready-to-use sterile, procedure-specific kits, which reduce setup complexity and risk. The move toward same-day surgeries and home healthcare also supports growth of prepackaged trays, since they simplify logistics, ensure consistency and ease sterilization compliance.
Regulators and payers are increasingly supporting the use of sterile, disposable medical kits and trays to lower costs associated with labor, infection control, and hospital stays. For example, in a U.S. study modelling total knee arthroplasty (TKA), use of single-use instruments saved nearly US $1,000 per case, largely by reducing costs of tray sterilization and improving operating room turnover.
At the same time, surgical site infections (SSIs) remain a major concern: in 2021-2022, eleven EU member states plus one European Economic Area (EEA) country reported over 10,000 SSIs from roughly 662,000 procedures, depending on procedure type. Governments are tying reimbursement and hospital accreditation to infection rates and mandating reporting of SSIs, which incentivizes hospitals to adopt standardized sterile disposables. Such policies reduce regulatory risk, labor for reprocessing instruments, and ultimately lead to more predictable costs, making prepackaged kits more attractive.
The growing use of prepackaged medical kits and trays, while improving efficiency and sterility, raises significant environmental concerns. Single-use disposable kits contribute substantially to medical waste, which requires specialized disposal to prevent contamination and toxin release. Improper handling leads to environmental pollution, including dioxins and microplastics, and increases overall healthcare costs due to waste management requirements. Studies indicate that healthcare facilities generate up to 5.9 million tons of medical waste annually in high-income countries, emphasizing the ecological and economic burden of widespread single-use kit adoption.
One major restraint in the prepackaged medical kits & trays market is the steep cost associated with specialized or customized designs, especially when advanced materials, disposables, proprietary sterilization and packaging are involved. For example, a Belgian cost-comparison study found that a full disposable instrument set for laparoscopic cholecystectomy was 7 to 27 times more expensive per procedure than using reusable instruments. Another study from German hospitals found that sterile container systems cost about €2.05 per use, while more complex wrapping systems raised costs to €3.87 per use. These elevated acquisition, packaging, and disposal costs are likely to hinder adoption in cost-sensitive healthcare settings.
Integrating the Internet of Things (IoT), smart packaging, and traceability technologies into prepackaged medical kits and trays presents a strong opportunity for both manufacturers and healthcare providers. Hospitals using Radio Frequency Identification (RFID)- or Ultra-wideband (UWB)-based tracking systems have reported major gains. For instance, one U.S. hospital reduced equipment search time from 30 minutes down to 5 minutes, boosting utilization rates by about 20%.
Smart containers and smart pallets with real-time data tracing help ensure that sterile trays maintain required conditions during storage and transport, lowering the risk of breach and enhancing sterility assurance. Additionally, asset tracking solutions reduce unnecessary purchases. Some institutions report a 20% decrease in overordering with IoT-enabled inventory systems. These technologies also support compliance with regulations needing audit trails (e.g. sterilization logs, usage history), making prepackaged kits more attractive in regulated settings and reducing labor/time burdens in tracking and record-keeping.
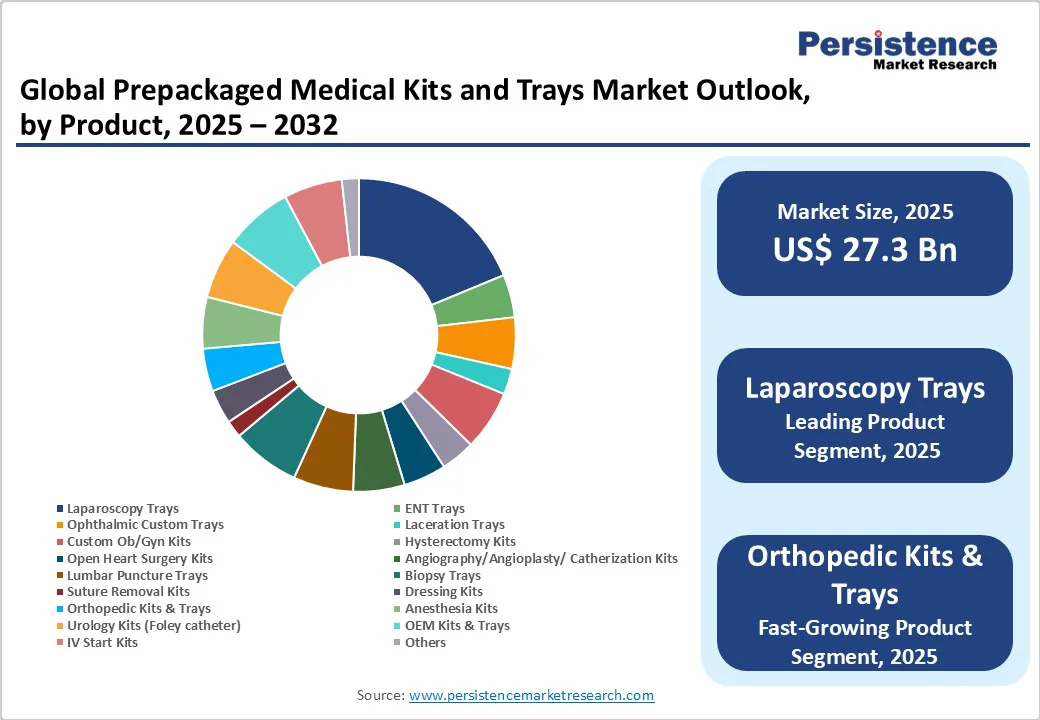
The laparoscopy trays segment is expected to hold the dominant share with 21.2% in 2025. This dominance stems from the rapid increase in minimally invasive laparoscopic procedures for general surgery, gynecology, and urology, which account for more than 15 million procedures annually worldwide (NIH, 2025). Laparoscopy offers benefits such as reduced hospital stay, faster recovery, and lower post-operative complications, making it the preferred option for both patients and providers.
Prepackaged laparoscopy trays are designed with standardized instruments and disposables that ensure sterility and reduce preparation time, which is crucial for high-volume surgical departments. Additionally, the growing prevalence of obesity and gastrointestinal disorders has driven up bariatric and abdominal laparoscopic surgeries. Combined with rising adoption in outpatient and day-care centers, laparoscopy trays are set to hold prominent revenue share, supported by both volume growth and higher per-procedure kit value.
Hospitals and clinics are projected to remain the leading end-user segment for prepackaged medical kits and trays, with nearly 45% by 2025. These facilities carry out the majority of surgical and diagnostic procedures, making them natural drivers of consistent and large-scale demand. Preassembled trays streamline surgical workflows by reducing preparation steps, ensuring that operating rooms run more efficiently and staff resources are better utilized.
The emphasis on maintaining strict infection-control protocols further encourages the use of sterile, single-use kits, which lower the risks associated with cross-contamination. Clinics, particularly those focusing on outpatient and day-care procedures, are also leaning heavily on prepackaged kits as they seek to manage growing patient volumes without compromising quality or cost-efficiency. Collectively, hospitals and clinics form the backbone of demand in this market, solidifying their position as the dominant revenue-generating segment.
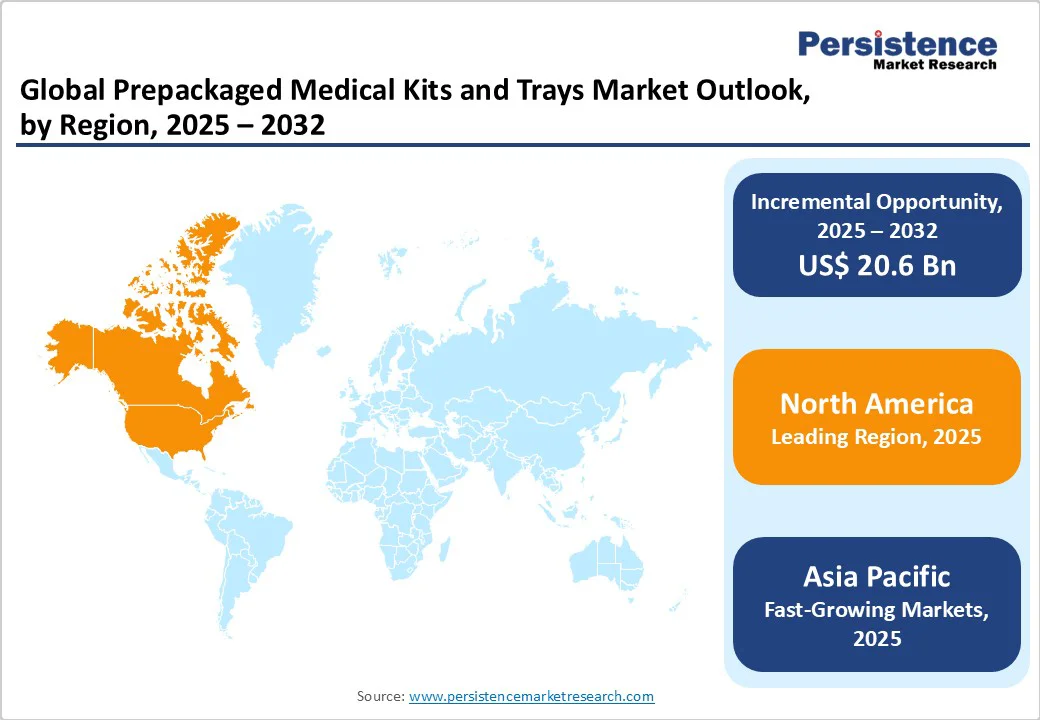
The North America Prepackaged Medical Kits & Trays market is expanding rapidly and is projected to account for 34.7% of the global market share by 2025. A major driver is the strong uptake of procedure-specific disposable kits across hospitals, ambulatory surgical centers (ASCs), and outpatient settings as providers strive for leaner workflows and lower risks. In the United States, there are now over 12,000 ASCs in operation (ASC Data, 2025), and both procedure volumes and facility numbers continue to rise. Outpatient settings have become the dominant site of care, accounting for more than 80% of surgeries nationwide.
ASCs, which offer same-day surgeries without overnight stays, benefit from favorable reimbursement models: Medicare pays less for procedures in ASC settings compared to hospital outpatient departments, making the shift economically attractive for payers and providers. The trend toward ambulatory care aligns with broader cost containment strategies in U.S. health policy.
Regulatory stringency in the U.S. and Canada demands high standards for sterility, traceability, and device safety, which encourages the adoption of ready-to-use sterile kits rather than in-house assembly. Academic and clinical literature emphasize that improper sterilization or reprocessing carries substantial risk, pushing institutions toward prepackaged sterile disposables.
Meanwhile, technological advances and procurement digitization are enabling customization of kits and just-in-time delivery, which reduces inventory burdens on hospitals and ASCs. Together, these dynamics make North America a fertile region for the expansion of prepackaged medical kits and trays.
Europe is a significant player in the global market, projected to hold around 29.4% share by 2025. This robust growth is primarily driven by stringent hygiene and infection control standards, coupled with stringent government quality regulations.
Widespread adoption of minimally invasive surgery (MIS), now considered the standard of care across Germany and much of Europe, is fueling the demand for specialized and custom trays tailored to specific surgical needs. Techniques such as single-incision laparoscopic surgery (SILS) reduce scarring, improve patient satisfaction, and enhance recovery, while micro- and nano-invasive procedures highlight the focus on surgical innovation. Prepackaged kits and custom trays complement these procedures by providing standardized, sterile instrument sets, improving workflow efficiency, and reducing errors.
In addition, initiatives like the NHS Supply Chain’s Custom Procedure Packs framework (June 2024–June 2026) exemplify Europe’s shift toward standardized and bespoke prepackaged kits. By consolidating surgical components into ready-to-use packs, this program enhances operational efficiency and cost-effectiveness across hospitals and healthcare networks.
The framework, involving 31 suppliers and a National Pricing Matrix, also encourages collaboration, allowing hospitals to contribute to the development of standardized packs through their Integrated Care System (ICS) Managers. Such efforts reflect a broader European trend of integrating efficiency, standardization, and innovation in surgical workflows, underscoring the region’s continued focus on improving patient outcomes while optimizing healthcare resources.
Asia Pacific is projected to experience rapid growth with a CAGR of around 10.5% in the prepackaged medical kits and trays market, driven by diverse factors across key countries. China prepackaged medical kits & trays market is witnessing significant expansion due to its robust healthcare infrastructure development and increasing demand for standardized surgical procedures. The government's focus on enhancing healthcare accessibility and quality, particularly in Tier-2 and Tier-3 cities, is fueling the adoption of prepackaged kits to streamline operations and ensure sterility.
Japan prepackaged medical kits & trays market stands out with its advanced medical technology and aging population. The country's emphasis on patient safety and efficiency in healthcare delivery is driving the demand for prepackaged medical kits and trays. Additionally, Japan's stringent regulatory standards ensure the adoption of high-quality, sterile products in medical settings.
India's prepackaged medical kits & trays market, with its burgeoning healthcare sector, is experiencing growth in medical tourism and an expanding middle class. The increasing awareness of infection control and the need for standardized procedures are propelling the demand for prepackaged kits. Moreover, cost sensitivity in the healthcare system presents opportunities for local manufacturing and assembly to meet the growing needs efficiently. Collectively, these countries are contributing to the dynamic growth of the APAC market, each with unique drivers aligning with regional healthcare priorities.
The global prepackaged medical kits and trays market is evolving with a focus on standardization, regulatory compliance, and operational efficiency. Industry developments emphasize innovation in sterile, procedure-specific kits, and collaborative approaches to enhance workflow, reduce procedural errors, and meet rising healthcare demands.
The global prepackaged medical kits & trays market is projected to be valued at US$ 27.3 Bn in 2025.
Rising surgical volumes and demand for sterile, time-saving solutions are driving adoption across hospitals and outpatient centers.
The global market is poised to witness a CAGR of 8.4% between 2025 and 2032.
Custom procedure kits, digital configurators, and affordable regional manufacturing offer strong growth opportunities in diverse care settings.
Major players in the global are Cardinal Health, Owens & Minor, Mölnlycke Health Care AB, PAUL HARTMANN AG, STERIS, Medline Industries, LP and others
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author