ID: PMRREP12475| 200 Pages | 23 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

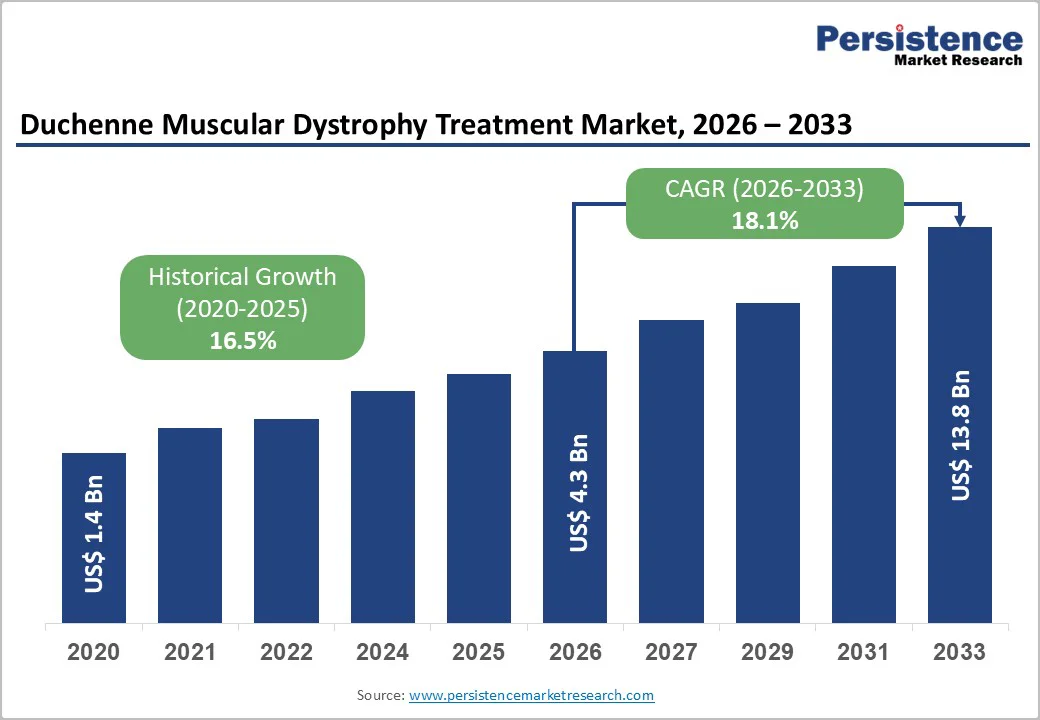
The global Duchenne muscular dystrophy treatment market size is estimated to grow from US$ 4.3 billion in 2026 to US$ 13.8 billion by 2033. The market is projected to record a CAGR of 18.1% from 2026 to 2033.
The market is steadily expanding, driven by growing patient awareness, rising adoption of molecular therapies, and advances in gene- and exon-skipping treatments. North America leads due to strong healthcare infrastructure, expertise, and reimbursement support. At the same time, the Asia Pacific is the fastest-growing region, fueled by improving access, awareness, and increasing investments in advanced DMD therapies.
| Key Insights | Details |
|---|---|
| Duchenne Muscular Dystrophy Treatment Market Size (2026E) | US$ 4.3 Bn |
| Market Value Forecast (2033F) | US$ 13.8 Bn |
| Projected Growth (CAGR 2026 to 2033) | 18.1% |
| Historical Market Growth (CAGR 2020 to 2025) | 16.5% |
Duchenne muscular dystrophy (DMD) is primarily a childhood-onset disease, affecting approximately 1 in every 3,500 to 5,000 live male births globally, with symptoms typically appearing between ages 2 and 6. The progressive nature of the disease often leads to loss of ambulation in childhood or early adolescence, making the pediatric population the entire addressable market for DMD therapies.
Systematic analyses estimate a global birth prevalence of around 19.8 per 100,000 live male births, and an overall prevalence of approximately 7.1 per 100,000 males. As diagnosis rates improve and supportive care extends patient survival, the demand for both established and novel treatments, including corticosteroids, gene therapy, exon-skipping therapies, and other supportive interventions, continues to rise.
This growing pediatric patient base directly drives market expansion, as early intervention is crucial for slowing disease progression, enhancing quality of life, and increasing the adoption of advanced therapies across global healthcare systems.
High treatment costs represent a major restraint for the market, limiting patient access and adoption. The first approved gene therapy for DMD, Elevidys, is priced at approximately $ 3.2 million per dose, making it one of the most expensive therapies worldwide. Exon-skipping therapies, such as Exondys 51, cost roughly $300,000- $500,000 annually per patient.
Even long-term management with corticosteroids, physiotherapy, and supportive care can accumulate to hundreds of thousands of dollars over a patient’s lifetime. These high expenses pose significant barriers, particularly in low- and middle-income regions, where health insurance coverage is limited or absent.
As a result, many patients delay treatment, limit therapy duration, or forgo advanced interventions entirely. The prohibitive cost not only affects patient outcomes but also constrains market expansion, as the majority of eligible patients cannot access these life-altering therapies, slowing the adoption of newer molecular and gene-based treatments despite their clinical efficacy.
Gene and exon-skipping therapies now offer, for the first time, the possibility of correcting the underlying genetic defect in DMD rather than just managing symptoms. The first gene therapy for DMD, Elevidys (delandistrogene moxeparvovec), received regulatory approval and delivers a micro-dystrophin gene with a single intravenous dose.
This breakthrough opens therapy to many patients with confirmed DMD mutations. Meanwhile, exon-skipping drugs such as Exondys 51 target a subset of mutations - about 13% of all DMD cases are amenable to skipping exon 51 alone, and broader analyses indicate that up to 55?% of DMD patients could benefit from exon-skipping approaches (e.g., exons 45, 51, 53, etc.).
This dramatically expands the pool of treatable patients. As more mutations become targetable and new molecular therapies emerge, the size of the addressable population grows substantially, offering a big opportunity for market growth worldwide.
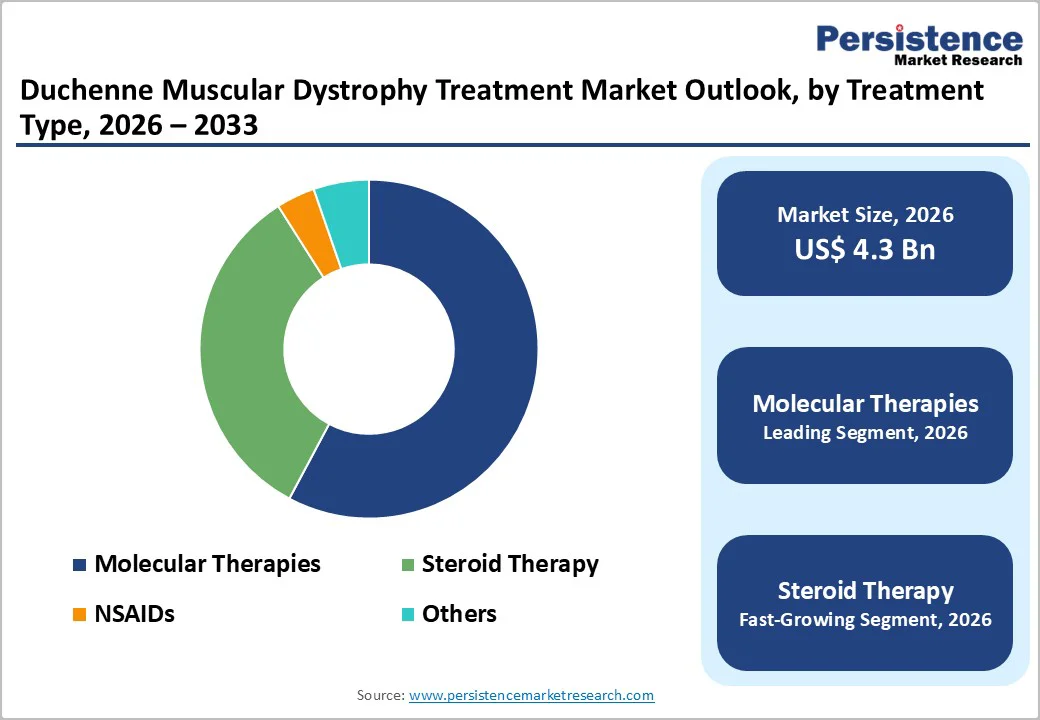
Molecular Therapies holds a 57.8% share of the global market in 2025, driven by superior clinical efficacy and the ability to address the underlying genetic cause of the disease. Gene therapies deliver functional dystrophin genes, offering long-term benefits with a single dose, while exon-skipping therapies target specific mutations, making treatment personalized and precise.
This mutation-specific approach provides broader patient coverage than conventional steroid therapy, which only manages symptoms. The combination of long-lasting effects, improved patient outcomes, and growing clinical acceptance positions molecular therapies as the leading treatment type in the global DMD market.
Oral administration dominates the Duchenne muscular dystrophy treatment market because most standard therapies, particularly corticosteroids such as deflazacort and prednisone, are administered orally. In 2023, oral drugs accounted for approximately two-thirds of the DMD treatment market, reflecting their widespread use and accessibility.
Oral therapy is especially suitable for pediatric patients, enabling daily at-home dosing without frequent hospital visits, thereby improving adherence and quality of life. Many supportive and early-stage treatments, including small-molecule drugs, are also oral, further reinforcing this preference.
The convenience, ease of administration, and ability to maintain consistent dosing make oral therapies the leading route of administration, capturing the largest share of the global DMD treatment market.
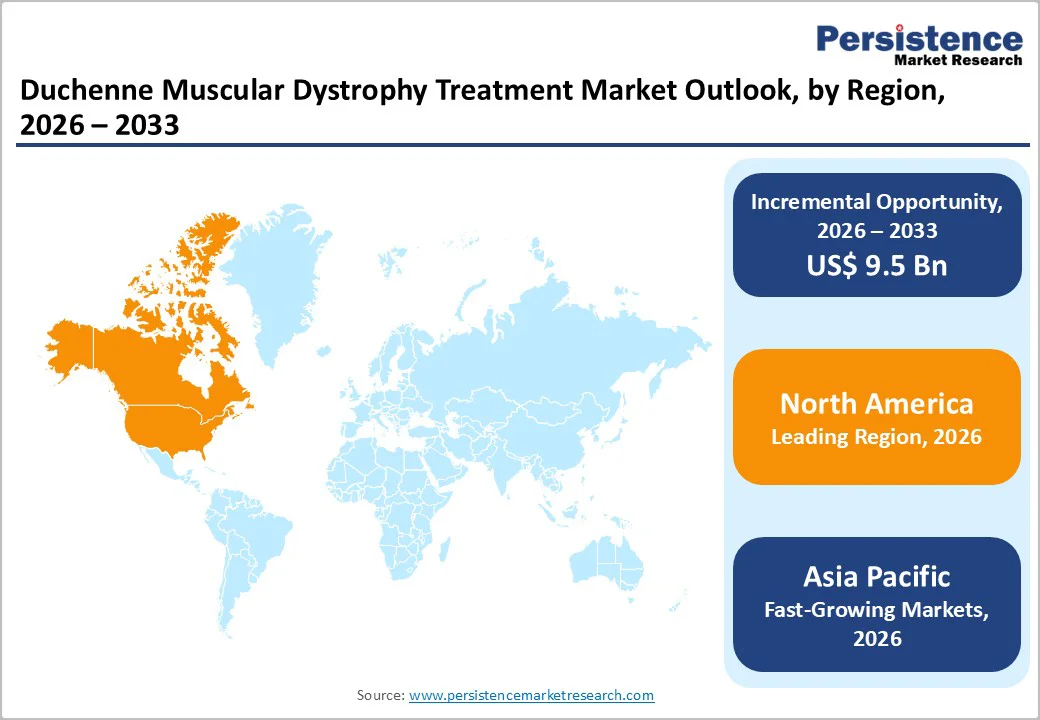
North America dominates the Duchenne muscular dystrophy treatment market with a 43.4% share in 2025, driven by high disease awareness, advanced healthcare infrastructure, and early access to approved therapies. In the U.S., comprehensive newborn screening, widespread access to genetic testing, and numerous specialized neuromuscular centers make diagnosis and ongoing care accessible.
The region accounts for nearly half of global therapy uptake and hosts the majority of clinical trials kits, ensuring fast access to novel gene and exon-skipping treatments. Furthermore, strong regulatory facilitation and reimbursement frameworks support patient access to expensive therapies, encouraging uptake. These factors together make North America the leading region for DMD treatment development and delivery.
Europe remains a key region for DMD because it combines a substantial patient population with strong public-health infrastructure and supportive regulatory frameworks. Across the European Union, about 26,000 people are estimated to live with DMD, making it one of the largest concentration zones globally for this rare disease.
Regulatory support from the European Medicines Agency (EMA), including orphan drug designations, helps accelerate the approval and access of new therapies. In many European countries, national health systems cover a substantial portion of treatment costs, improving affordability and uptake.
Additionally, coordinated networks such as EURO-NMD integrate clinical centers across >20 countries to support diagnostics, care guidelines, and data sharing, thereby enhancing early diagnosis rates and comprehensive care delivery. These elements make Europe a pivotal market for both existing and emerging DMD therapies.
Asia Pacific is seeing rapid growth in DMD treatment adoption because its large population base, combined with improving healthcare infrastructure, is uncovering many previously undiagnosed cases. Meta-analytic data show that muscular dystrophies in Asia have a prevalence comparable to global averages, meaning a substantial absolute number of patients.
Meanwhile, countries such as China and India have boosted health-care spending: health-expenditure-to?GDP ratios have risen significantly over the past decade, enabling the expansion of diagnostic and treatment facilities. Concurrently, increased government focus on rare diseases and rising disease awareness are driving greater uptake of novel therapies.
Together, these trends, including population size, improved diagnostics, rising healthcare spending, and growing awareness, make Asia Pacific the fastest expanding region for DMD treatments.
Leading companies in the Duchenne muscular dystrophy treatment market focus on developing advanced molecular and gene therapies, investing in R&D, clinical trials, and staff training. They collaborate with hospitals and research centers to enhance treatment precision, improve safety, and expand global adoption, addressing the growing demand for effective, targeted, and mutation-specific DMD therapies worldwide.
The global duchenne muscular dystrophy treatment market is projected to be valued at US$ 4.3 Bn in 2026.
Rising patient awareness, advances in gene and exon-skipping therapies, pediatric care focus, supportive regulations, and increasing diagnosis rates drive the market.
The global Duchenne muscular dystrophy treatment market is poised to witness a CAGR of 18.1% between 2026 and 2033.
Expansion of gene and exon-skipping therapies, emerging market growth, pediatric-focused treatments, affordable options, and collaborations drive Duchenne muscular dystrophy market opportunities.
Sarepta Therapeutics, PTC Therapeutics, Santhera Pharmaceuticals, Italfarmaco S.p.A., NS Pharma, Inc., Capricor Therapeutics, Inc.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Treatment Type
By Route of Administration
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author