ID: PMRREP15679| 210 Pages | 20 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

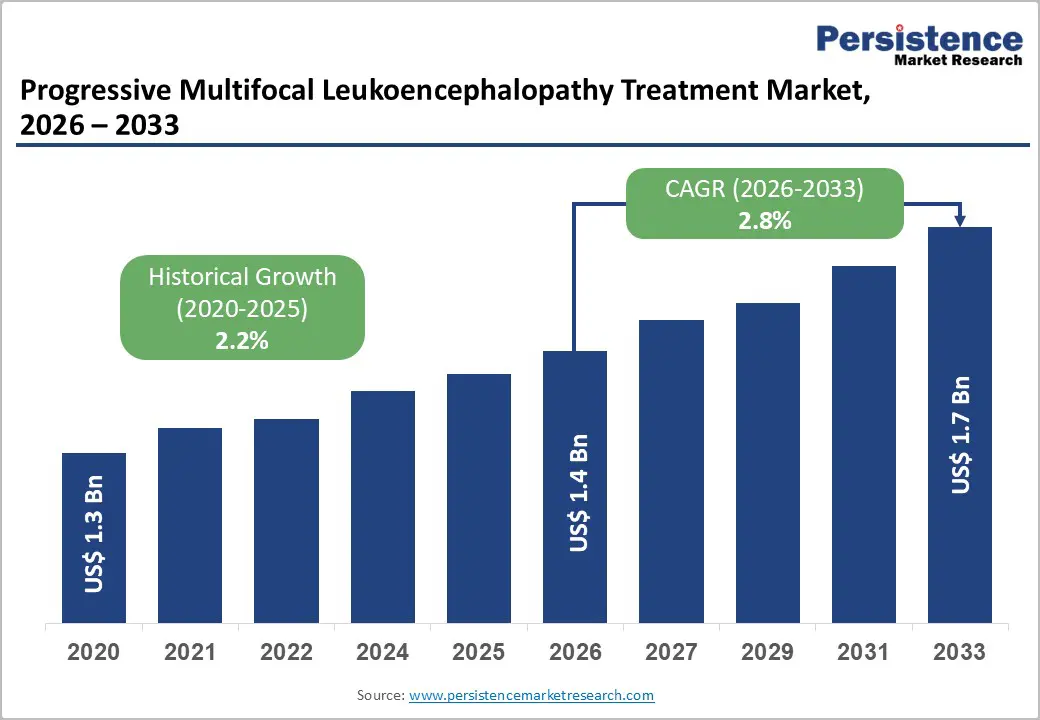
The global progressive multifocal leukoencephalopathy treatment market size is likely to be valued at US$ 1.4 billion in 2026 and US$1.7 billion by 2033, growing at a CAGR of 2.8% during the forecast period from 2026 to 2033.
The global market is growing gradually, driven by rising immunocompromised populations, increased HIV prevalence, and improving diagnostic awareness. North America leads due to advanced healthcare infrastructure and higher diagnosis rates, while Asia-Pacific shows the fastest growth, supported by expanding healthcare access, improving treatment availability, and growing awareness of rare neurological disorders.
| Key Insights | Details |
|---|---|
|
Progressive Multifocal Leukoencephalopathy Treatment Market Size (2026E) |
US$ 1.4 Bn |
|
Market Value Forecast (2033F) |
US$ 1.7 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
2.8% |
|
Historical Market Growth (CAGR 2020 to 2025) |
2.2% |
The rising demand for accurate, high-throughput analytical techniques like LC-MS is closely tied to expanding pharmaceutical and biotechnology R&D activity. Governments and industry groups report substantial and growing investment in drug R&D; for example, the pharmaceutical industry spent approximately USD 129 billion in R&D in 2021, with sustained increases over the past decade, particularly in OECD countries and emerging markets such as China, where R&D expenditure grew markedly from 2010 to 2022. This intense R&D focus reflects the need for precise analytical tools capable of detecting trace impurities, characterizing complex molecules, and supporting regulatory compliance throughout drug discovery and development pathways.
The global burden of immunocompromised populations, especially people living with HIV (PLWH), illustrates the scale of this driver. According to recent data, over 38 million people worldwide are estimated to be living with HIV, a condition that inherently compromises immune function and increases susceptibility to opportunistic infections like PML. In the United States alone, approximately 1.2 million people are PLWH, and even with ART, many remain at risk due to historically low CD4 counts or incomplete immune restoration. As HIV and other causes of immunosuppression (e.g., cancer, transplant therapy, autoimmune disease) persist or grow, the pool of individuals vulnerable to PML expands, reinforcing the role of immunocompromise as a primary market driver for PML treatment.
Progressive multifocal leukoencephalopathy carries a notably poor prognosis, making mortality and outcome uncertainty a significant restraint on the treatment market. Historically, before effective HIV therapy, median survival after PML diagnosis was approximately six months, and most patients died within two years. Even with modern care, 30–50 percent of patients die within the first few months after diagnosis, and many survivors are left with severe neurological deficits. This high fatality rate reflects the aggressive nature of JC virus–induced brain damage and the absence of widely effective targeted therapies.
Reliable clinical studies emphasize the persistence of poor long-term outcomes, imposing challenges for clinicians and limiting market growth potential. Research reports an overall PML mortality rate of 74.5–78.0 percent in HIV populations and up to 90 percent case-fatality in non-HIV groups, despite antiretroviral therapy improvements. Additionally, one-year survival often remains below 60 percent without strong immune recovery. These statistics underscore how high mortality and severe disability rates constrain treatment adoption and research investment, as effective interventions remain elusive and prognosis remains guarded even with the best available care.
Progressive Multifocal Leukoencephalopathy (PML) is caused by reactivation of the JC virus (JCV) a ubiquitous polyomavirus present in 50–80 percent of adults worldwide in latent form. Because JCV reactivation in immunocompromised individuals drives PML pathogenesis, development of direct antivirals targeting viral replication or entry mechanisms represents a key therapeutic opportunity. Current treatment options primarily focus on restoring immune function rather than blocking viral replication, and no direct-acting antiviral drug has yet been approved for JCV infection or PML. This unmet need highlights the potential for innovative antiviral therapies that can specifically interrupt the JCV life cycle and reduce viral burden in afflicted patients.
Reliable medical guidelines and research underscore the absence of effective direct antivirals against JCV, emphasizing the critical need for targeted therapies. Clinical guidance notes that although various antiviral agents (e.g., cytarabine, cidofovir) have been explored, none are recommended due to lack of effectiveness, and direct-acting antivirals for JCV remain unavailable. This gap persists despite high JCV seroprevalence (20–70 percent in adults by serology) and significant viral loads in PML patients’ cerebrospinal fluid, correlating with disease severity. The combination of high latent infection rates and the absence of specific antiviral treatments creates a substantial opportunity for the development of JC virus-targeted agents that could transform PML care and improve outcomes.
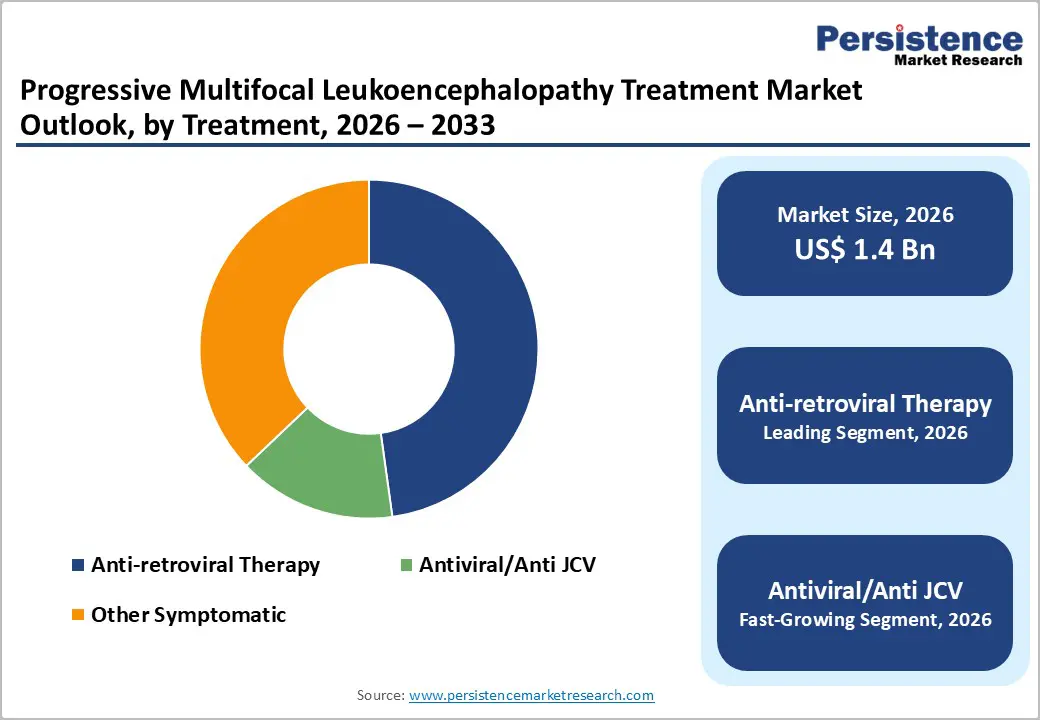
Anti-retroviral Therapy dominates with 45.1% share of the global market in 2025, because a substantial proportion of PML cases occur in people living with HIV/AIDS. Before widespread ART use, 3–7 percent of individuals with AIDS developed PML, and HIV remains the most common condition associated with PML globally. ART restores immune function by suppressing HIV replication, increasing CD4 counts, and reducing opportunistic infections; studies show that immune recovery with ART significantly improves PML survival and neurological outcomes. In cohorts treated with ART, survival rates at one year have improved compared with pre-ART eras, reflecting the central role of immune reconstitution rather than direct antiviral agents against the JC virus. As a result, ART is the predominant therapeutic approach in PML management.
HIV/AIDS dominates the progressive multifocal leukoencephalopathy treatment market by indication because it represents the largest and most established risk group for PML development. PML is caused by reactivation of the John Cunningham virus in settings of profound immune suppression, which is most commonly seen in advanced HIV infection. Clinical data indicate that approximately 80–85% of PML cases occur in people living with HIV/AIDS, making it the leading underlying condition associated with the disease. Before the widespread use of antiretroviral therapy, 3–7% of patients with AIDS developed PML, and it was among the most frequent opportunistic neurological infections. Although ART has reduced incidence, untreated or late-diagnosed HIV continues to drive PML burden globally, sustaining HIV/AIDS as the dominant indication segment.
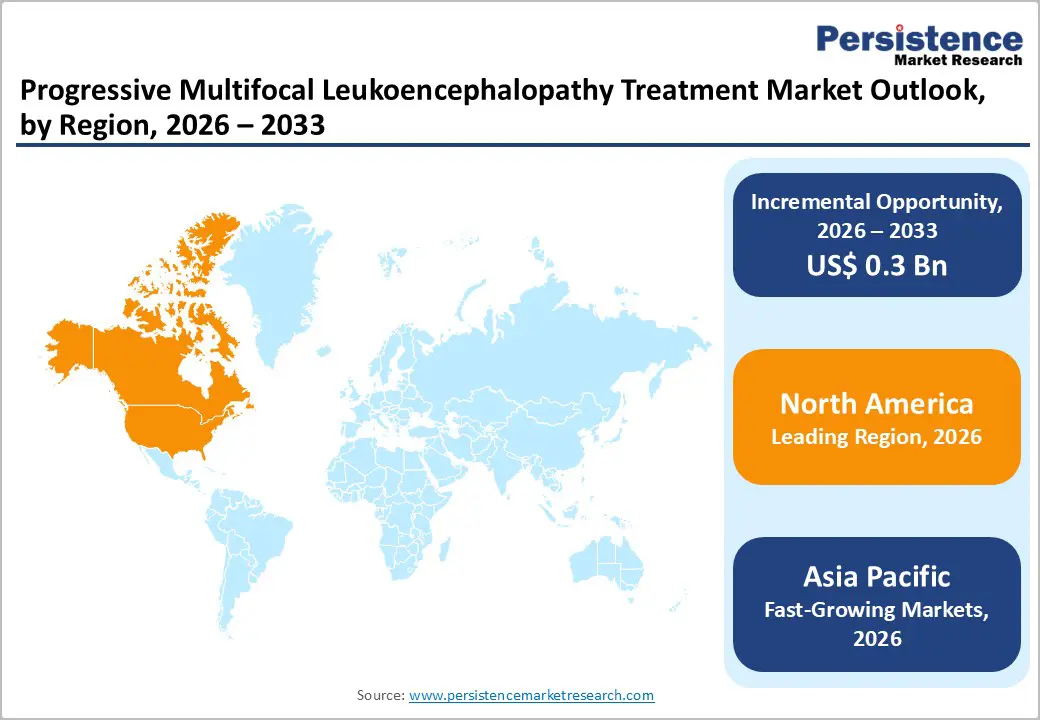
North America dominates the progressive multifocal leukoencephalopathy treatment market with 39.1% share in 2025, due to its advanced healthcare infrastructure and high-quality medical services. The region has well-established diagnostic capabilities, including widespread availability of MRI and cerebrospinal fluid testing, which enable early detection of PML in immunocompromised patients, particularly those living with HIV/AIDS or undergoing immunosuppressive therapy. The U.S. alone has over 1.2 million people living with HIV, representing a significant at-risk population for PML. Furthermore, North America benefits from specialized neurology and infectious disease centers, strong access to antiretroviral therapy, and comprehensive patient monitoring programs. These factors together ensure better identification, management, and adoption of treatment, explaining the region’s leadership in the PML treatment domain.
Europe is an important region in the progressive multifocal leukoencephalopathy treatment landscape due to its high standards of healthcare, extensive immunocompromised populations, and strong emphasis on rare disease surveillance. Countries in the European Union maintain robust registries for HIV/AIDS and multiple sclerosis (MS), two major conditions associated with PML. For example, the European Centre for Disease Prevention and Control (ECDC) reports that the EU/EEA has over 2.2 million people living with HIV, many of whom are monitored closely for opportunistic infections. Additionally, MS prevalence in Europe is among the highest globally, with up to 200 cases per 100,000 people in some countries, increasing exposure to immunomodulatory therapies that can elevate PML risk. With advanced diagnostic imaging and well-established healthcare access, Europe’s focus on integrated care pathways enables timely detection and management of PML, reinforcing its strategic importance for understanding disease burden and optimizing therapeutic responses across diverse patient populations.
Asia Pacific is the fastest-growing region for progressive multifocal leukoencephalopathy treatment due to expanding healthcare access, rising at-risk populations, and improved diagnostic capacity. The region carries a significant share of the global HIV burden, with the UNAIDS 2023 report estimating about 6.2 million people living with HIV in Southeast and East Asia, where immunosuppression increases PML risk. Many countries are strengthening healthcare systems; for instance, India’s healthcare expenditure rose from 3.5% of GDP in 2019 to over 4% in 2024, enhancing access to advanced imaging and neurological care. Additionally, increasing use of immunosuppressive therapies for cancers and autoimmune diseases elevates PML awareness and case identification. As diagnostic infrastructure, clinical training, and treatment availability improve, more PML cases are detected and managed, driving the regional growth.
Leading PML treatment stakeholders focus on improving early diagnosis, immune restoration, and patient monitoring. By enhancing detection accuracy, treatment outcomes, and care coordination, they support HIV/AIDS, transplant, and immunosuppressive patient management, streamline clinical decision-making, and enable better survival. These advancements drive adoption of therapies, expand access, and fuel growth in the global market.
The global progressive multifocal leukoencephalopathy treatment market is projected to be valued at US$ 1.4 Bn in 2026.
Rising immunocompromised populations, increasing HIV/AIDS prevalence, improved diagnostics, expanding immunosuppressive therapies, and growing awareness drive PML treatment market growth.
The global progressive multifocal leukoencephalopathy treatment market is poised to witness a CAGR of 2.8% between 2026 and 2033.
Development of JC virus-targeted antivirals, advanced diagnostics, immunotherapy strategies, personalized treatment, and expansion in emerging healthcare markets create key PML opportunities.
Pfizer, Inc., F. Hoffman - La Roche Ltd., Gilead Sciences, Inc., GSK, Sanofi S.A., Allergan plc.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Treatment
By Indication
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author