ID: PMRREP35325| 200 Pages | 16 May 2025 | Format: PDF, Excel, PPT* | Healthcare

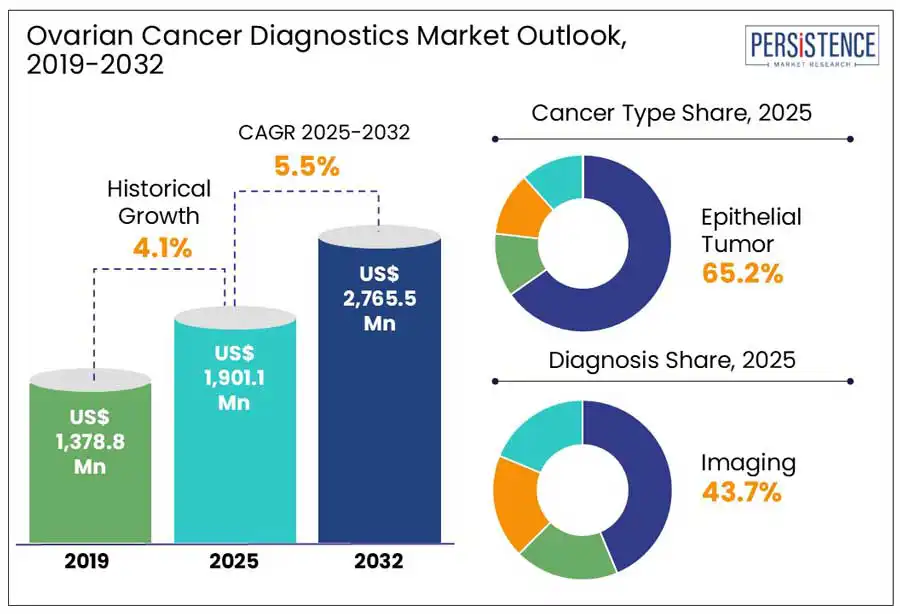
The global ovarian cancer diagnostics market size is predicted to reach US$ 2,765.5 Mn in 2032 from US$ 1,901.1 Mn in 2025. It will likely witness a CAGR of around 5.5% in the forecast period between 2025 and 2032.
Ovarian cancer diagnostics has become a key focus in women’s healthcare owing to the high mortality and the disease’s asymptomatic progression rate when detected at advanced stages. The diagnostic landscape is constantly evolving, spurred by the integration of innovative liquid biopsy technologies and molecular biology. Growth in the ovarian cancer segment is also directly influencing innovations in the women’s health imaging system market, finds Persistence Market Research. Key companies are expected to develop unique CT, MRI, and transvaginal ultrasound technologies to help improve staging accuracy and early detection of ovarian cancer.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Ovarian Cancer Diagnostics Market Size (2025E) |
US$ 1,901.1 Mn |
|
Market Value Forecast (2032F) |
US$ 2,765.5 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.1% |
Increasing prevalence of ovarian cancer across the globe is predicted to bolster the ovarian cancer diagnostics market growth through 2032. The high mortality rate of the disease and the limitations of current screening methods are also contributing to market growth. As per GLOBOCAN 2024 estimates, more than 325,000 new ovarian cancer cases were diagnosed worldwide, with approximately 210,000 deaths. These numbers are estimated to rise significantly in the forecast period, highlighting the urgent requirement for early detection methods.
Demand will likely remain particularly high in low- and middle-income countries, where aging demographics, declining fertility rates, and lifestyle changes are surging incidence rates. These trends are anticipated to pressurize healthcare systems to prioritize diagnostic innovation for early-stage detection, where survival rates are significantly high. In emerging countries, government-backed initiatives are projected to play key roles in extending early cancer screening coverage.
High false-positive and false-negative rates are predicted to hinder the adoption and clinical reliability of ovarian cancer diagnostics. This is evident in early-stage detection, where high precision is important. Even though conventional CA-125 markers are widely used, these are elevated in multiple benign conditions such as pelvic inflammatory disease and endometriosis. As per a 2023 report published by the U.S. Preventive Services Task Force, routine screening with CA-125 in asymptomatic women often results in unnecessary surgical interventions. This is because false positives contribute to overtreatment and overdiagnosis. It not only raises healthcare costs but also declines patient trust in existing diagnostic tools.
False negatives are also speculated to result in missed early-stage diagnoses when survival rates surpass 90%. The University of California recently found that with the use of traditional dual-marker strategies, up to 30% of early-stage ovarian cancers went undetected in women with low-grade serous tumors. These limitations are anticipated to directly affect the diagnostic biomarker market. Researchers are hence striving to validate innovative biomarkers in more diverse populations and across a wide range of tumor subtypes. They aim to address variability in test performance.
In the cancer diagnostics market, including ovarian cancer, biomarker research is expected to play a significant role in transforming the landscape by enabling accurate and early detection through molecular profiling. Recent breakthroughs have shifted the focus beyond traditional markers such as HE4 and CA-125 to innovative biomarkers, including exosomal proteins, ctDNA, and microRNAs.
For example, a 2024 study published in Nature Communications detected a panel of five microRNAs that showed more than 90% specificity and sensitivity in differentiating between benign pelvic masses and early-stage ovarian cancer. Similar discoveries are poised to propel commercial interest in integrating multi-analyte biomarker panels into diagnostic workflows, offering a reliable diagnostic method.
Based on cancer, the market is trifurcated into epithelial tumor, germ cell tumor, and stromal cell tumor. Out of these, epithelial tumor is expected to generate approximately 65.2% of the ovarian cancer diagnostics market share in 2025. These tumors are considered the most commonly detected subtype in diagnostic screenings. A 2024 study published in The Lancet Oncology, for instance, showed that more than 87% of the 2,500 women who received a standard screening diagnosis had epithelial subtypes. This indicates both biological prevalence and the inherent bias existing testing protocols. The gynecological cancer drugs market is also expected to be driven by the robust therapeutic and diagnostic alignment associated with epithelial tumors. Pharmaceutical companies are investing in epithelial-specific drug targets, including folate receptor alpha (FRα), with recent approvals from government bodies.
Germ cell tumors are rare and account for only 5% of all ovarian cancers. They have become a key focus of leading companies, backed by their high prevalence in adolescents and unique clinical profile. These tumors are usually present in women aged 10 to 30, unlike epithelial tumors that mainly affect postmenopausal women. The curability of germ cell tumors with chemotherapy, specifically cisplatin-based regimens, has made precise and early diagnostics important to treatment planning. Existing diagnostic platforms are being innovated to include germ cell-specific panels, especially in young adult and pediatric oncology settings. This is because in such settings, treatment decisions rely on biomarker profiling and histological accuracy.
In terms of diagnosis, the market is segregated into imaging, blood test, and biopsy. Among these, the imaging segment will likely hold a share of around 43.7% in 2025. It is considered one of the most preferred modalities in the cancer tissue diagnostics market. This is due to its real-time anatomical visualization and non-invasive nature. Transvaginal Ultrasound (TVUS) is mainly favored for initial screening, mainly in those with pelvic masses or in symptomatic women. The TVUS technique is also easily accessible and relatively low-cost, making it a significant tool in middle- and low-income countries. Innovative imaging modalities, including contrast-enhanced CT scans and MRI, are also used to improve diagnosis. It is mainly evident in complex cases, where metastasis, lymph node involvement, and tumor morphology require assessment.
Blood tests, on the other hand, are envisioned to exhibit a steady CAGR from 2025 to 2032. It is due to their ability to detect tumor-associated biomarkers that provide early indications of malignancy. Recent developments have extended the role of blood tests beyond CA-125. The inclusion of human epididymis protein 4 (HE4) as part of the Food and Drug Administration (FDA)-approved Risk of Ovarian Malignancy Algorithm (ROMA) has enhanced diagnostic accuracy in separating malignant from benign pelvic masses. The surge of liquid biopsy techniques is also predicted to bolster blood-based diagnostics. This is because liquid biopsies are capable of detecting ovarian cancer at early stages by assessing tumor DNA or microRNAs.
In North America, the U.S. ovarian cancer diagnostics market will likely outpace the other countries by accounting for a share of about 41.6% in 2025. This is because of the rapid integration of Artificial Intelligence (AI) in diagnostic imaging and the early adoption of risk-based screening protocols. As per the American Cancer Society, nearly 19,680 new ovarian cancer cases were predicted in the U.S. in 2024 alone. It emphasized the rising demand for innovative early detection tools and high diagnostic accuracy.
The AI in oncology market is speculated to see developments in the U.S., especially in the ovarian cancer segment. Researchers at Johns Hopkins, for example, recently developed the DELFI-Pro test. It combines protein biomarkers HE4 and CA-125 with AI analysis of cell-free DNA fragmentation patterns. With a 72% detection rate in stage I cases, this approach has exhibited improved detection rates across all stages of ovarian cancer. The Georgia Tech Integrated Cancer Research Center has also launched an AI-based diagnostic test that assesses blood metabolites to locate ovarian cancer with around 93% accuracy. This probabilistic, personalized approach provides a more improved assessment compared to conventional binary tests.
In Europe, the U.K. is currently witnessing considerable growth in ovarian cancer diagnostics due to the development of unique approaches aimed at enhanced patient outcomes and early detection. A key development is the ROCkeTS study in the country. It was discovered that more than 25% of women with high-grade serous ovarian cancer can receive early diagnoses through symptom-triggered testing. It is predicted to result in a five-year survival rate of 93% in early-stage cases.
Similarly, the University of Oxford has been developing the world’s first ovarian cancer vaccine called OvarianVax. It will likely be developed to train the immune system to detect and attack early-stage ovarian cancer cells. In terms of the cell-based immunotherapy market, Europe is showcasing significant progress. GSK's combination of Jemperli with standard chemotherapy and Zejula, for instance, has indicated enhanced progression-free survival in patients with advanced ovarian cancer. Genmab's acquisition of ProfoundBio for US$ 1.8 Bn also improved its oncology portfolio. It included an antibody-drug conjugate for ovarian cancer called Rina-S.
Asia Pacific is expected to see significant developments in ovarian cancer diagnostics, propelled by increasing focus on early detection, government initiatives, and technological innovations. A pioneering clinical trial led by Professor Carlos Salomon Gallo at the University of Queensland in Australia, for example, is currently scrutinizing a unique blood test. It targets Extracellular Vesicles (EVs) for early-stage ovarian cancer detection. The test has already exhibited a 94% accuracy rate with a low false positive rate of 4%. It provides a promising tool for early diagnosis among postmenopausal women over 45 without prior ovarian cancer history.
In India, a partnership between the Indian Institute of Technology-Madras and the Cancer Institute has resulted in the development of a point-of-care, affordable diagnostic kit that uses blood samples. Such initiatives aim to enhance outpatient diagnosis, mainly benefiting resource-limited settings. In China, the market is predicted to be driven by the rising prevalence of ovarian cancer, pushing investments in diagnostic tools. A study published in BMC Women's Health, for instance, stated a fast-increasing trend in both mortality and incidence rates in China. In response, the country is speculated to focus on early treatment and detection strategies.
The ovarian cancer diagnostics market is characterized by intense innovation-driven rivalry. Leading companies are focusing on coming up with multi-modal diagnostic approaches that blend AI-based algorithms, imaging, and biomarkers. Conventional HE4 and CA-125-based diagnostics are being improved with proteomics-driven tests and next-generation liquid biopsies.
Start-ups are striving to target early detection, which is still a niche area in ovarian cancer care. They are pioneering non-invasive diagnostics by using innovative exosome-based technologies and biomarker panels. A few other players are aiming to gain fast-track approvals from regulatory bodies to market their products globally.
The market is projected to reach US$ 1,901.1 Mn in 2025.
The increasing prevalence of ovarian cancer and rising demand for early detection tools are the key market drivers.
The market is poised to witness a CAGR of 5.5% from 2025 to 2032.
Surging development of next-generation biomarker panels and increasing innovations in imaging technologies are the key market opportunities.
F. Hoffmann-La Roche AG, AstraZeneca Plc, and Johnson & Johnson Services Inc. are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Cancer Type
By Diagnosis
By End Use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author