ID: PMRREP22552| 198 Pages | 29 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

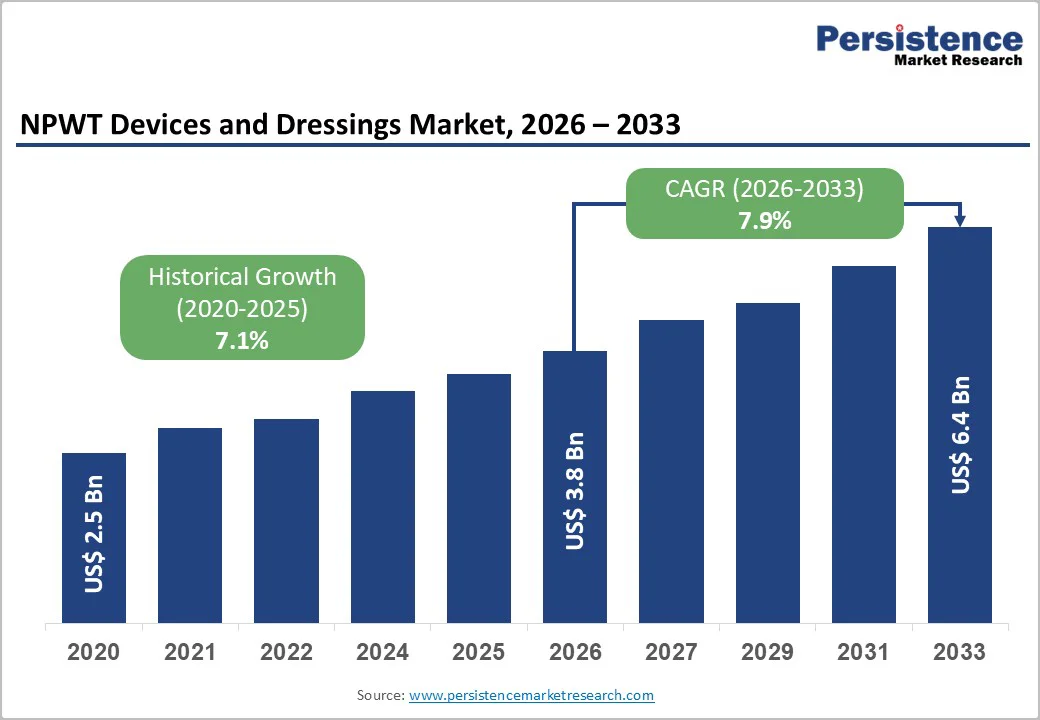
The global NPWT devices and dressings market size is projected to value at US$3.8 billion in 2026 to US$6.4 billion by 2033, growing at a CAGR of 7.9% during the forecast period from 2026 to 2033. The healthcare industry is evolving toward more portable, digitally enabled wound care systems that support faster healing and reduced infection risks.
The increasing incidence of chronic wounds, diabetic ulcers, trauma injuries, and post-surgical complications continues to drive adoption. Modern NPWT units offer adjustable pressure modes, exudate monitoring, and single-use options, improving patient mobility and enabling home-based treatment. Dressings are shifting toward antimicrobial foams, hydrofiber materials, and enhanced sealing technologies to optimize granulation and moisture balance. Growing hospital-to-home transition, reimbursement support in advanced markets, and greater awareness among clinicians are expanding utilization across acute care, long-term care centers, and ambulatory settings.
| Key Insights | Details |
|---|---|
|
NPWT Devices and Dressings Market Size (2026E) |
US$3.8 Bn |
|
Market Value Forecast (2033F) |
US$6.4 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
7.9% |
|
Historical Market Growth (CAGR 2020 to 2024) |
7.1% |
Rapid early-discharge protocols after surgery are strongly influencing the uptake of NPWT systems because healthcare providers are restructuring surgical recovery pathways to reduce the length of hospital stay without compromising healing quality. In orthopedic, bariatric, abdominal, spine, and cardiovascular procedures, wound integrity during the first week is critical, especially where sutures or staples are used under high-tension skin closure. Hospital administrators are now prioritizing faster transitions to home care or step-down facilities, typically within 2–5 days, and portable NPWT systems play a central enabling role. These lightweight systems apply controlled negative pressure to stabilize incision edges, reduce seroma formation, lower exudate accumulation, and minimize bacterial infiltration.
As a result, wound reopening rates decrease substantially, directly lowering hospital readmission penalties. Families also perceive portable NPWT devices as safer and more manageable than frequent dressing changes, thereby allowing caregivers to participate in wound monitoring actively. This shift simultaneously frees high-demand post-operative beds, improves operating room throughput, and supports reimbursement-linked quality metrics tied to infection control, making early-discharge-driven NPWT adoption increasingly strategic for hospitals.
Restrictive infection-control protocols significantly limit wider use of NPWT systems, especially in highly sterilized surgical environments. Operating theatres, transplant units, burn ICUs, and post-graft recovery wards maintain extremely stringent hygiene standards to prevent cross-contamination and microbial exposure. NPWT units introduce additional complexities because they involve canisters collecting biological fluids, exposed external tubing, and dressings that must create a fully sealed negative-pressure environment. These components require careful disposal, sterilization, and documented maintenance, adding to nursing workload. For instance, frequent canister changes and disinfection of connection points are mandatory to avoid bacterial proliferation.
Similarly, if the seal fails even slightly, air leaks introduce microbial risks and compromise wound protection. Hospitals must also segregate NPWT waste streams and comply with biomedical disposal norms, which creates additional logistical steps. Nurses must perform skill-based dressing application, and prolonged therapy often exceeds the routine change schedule recommended in infection-critical zones. This complexity discourages clinicians from prescribing NPWT for routine postoperative wounds, limiting its use to critical or non-healing cases where the benefits justify the procedural burden.
Smart dressings equipped with leakage and saturation alert technology represent a transformative opportunity in NPWT care. Leakage detection remains a major complication in wound sealing, often leading to pressure loss, exposure to infection, increased dressing changes, and prolonged healing. By embedding miniaturized biosensors or conductive micro-layers into dressings, clinicians can receive real-time alerts when seal integrity deteriorates, exudate volume exceeds capacity, or moisture dispersion becomes uneven.
Data transmission through Bluetooth or cloud-based dashboards allows caregivers to intervene early, especially in home-care settings where trained supervision is limited. This feature also reduces unnecessary dressing changes, minimizing costs and improving patient comfort. Additionally, automated digital logs enable trend evaluation, supporting decisions on suction pressure adjustments or dressing selection. For hospitals, such systems enhance compliance documentation and reduce readmission risk associated with wound failures. As remote wound care expands, smart dressings will become a high-value differentiator, accelerating healing outcomes and improving workflow efficiency.
NPWT Dressing Kits account for the largest share, mainly because they have a recurring consumption cycle throughout the treatment duration. Unlike devices, which are purchased once and used repeatedly, dressing kits must be changed at regular intervals based on wound severity, exudate levels, and sealing efficiency. A typical NPWT patient requires multiple dressing changes every week, resulting in higher cumulative usage per device installed. Hospitals, wound-care clinics, and home-care providers continuously procure replenishment kits, making consumables a predictable and ongoing spend category. These kits also come in multiple variants: foam-based, hydrofiber, antimicrobial, or incisional dressings, expanding product combinations and increasing revenue opportunities.
Moreover, dressing kits represent procedural billing components, allowing providers to charge for each replacement, further boosting utilization. In chronic wounds, such as diabetic foot ulcers or venous leg ulcers, treatment extends over several weeks or months, multiplying dressing consumption. Therefore, continuous turnover, broader SKU coverage, and greater volume dependence make NPWT dressing kits the dominant revenue contributor relative to NPWT device hardware purchases.
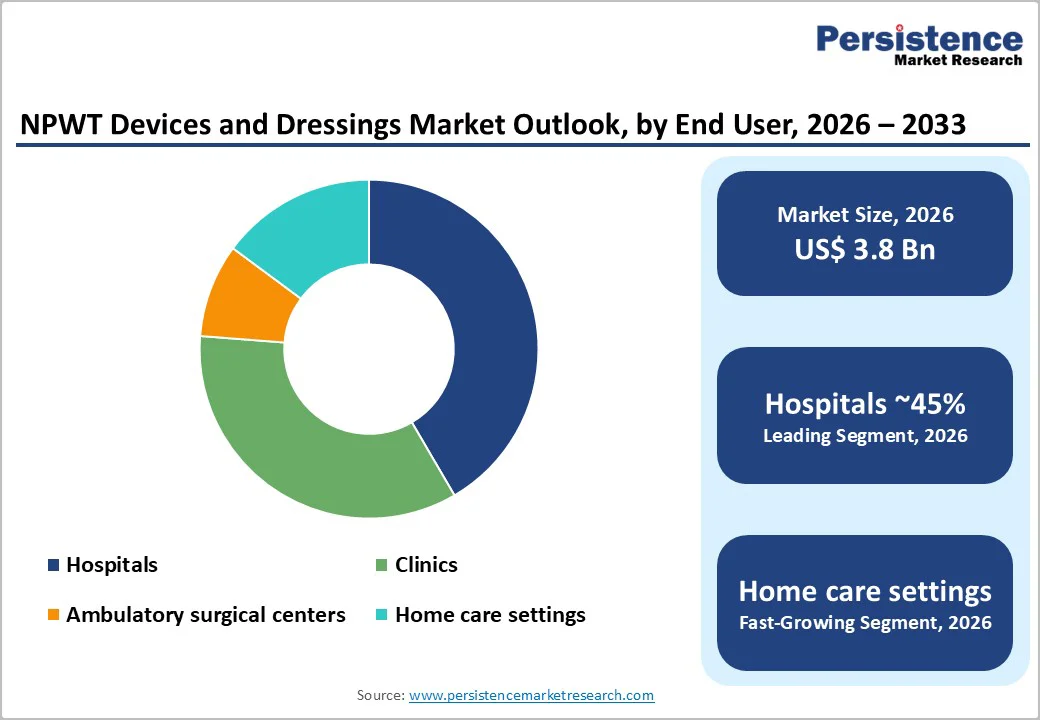
Hospitals hold the dominant share because most NPWT therapies are initiated in acute and complex wound settings, such as post-operative surgeries, trauma injuries, diabetic foot ulcers, and graft stabilization. NPWT initiation typically requires physician supervision, wound assessment, fluid volume estimation, and pressure calibration steps usually performed in hospital wound-care units or surgical departments.
Hospitals manage a higher volume of severe and high-risk patients, making NPWT adoption routine within orthopedic, cardiovascular, and plastic surgery cases. Also, reimbursement claims, electronic records, and infection-control protocols are streamlined in hospital setups, encouraging greater usage of both devices and dressing kits. Once therapy is started in hospitals, patients may be transferred to home care for continued treatment. Still, the highest procurement volume originates from hospitals, which purchase systems in bulk, maintain inventories, and standardize NPWT in treatment guidelines. Hence, hospitals represent the largest and most revenue-intensive user segment in this market.
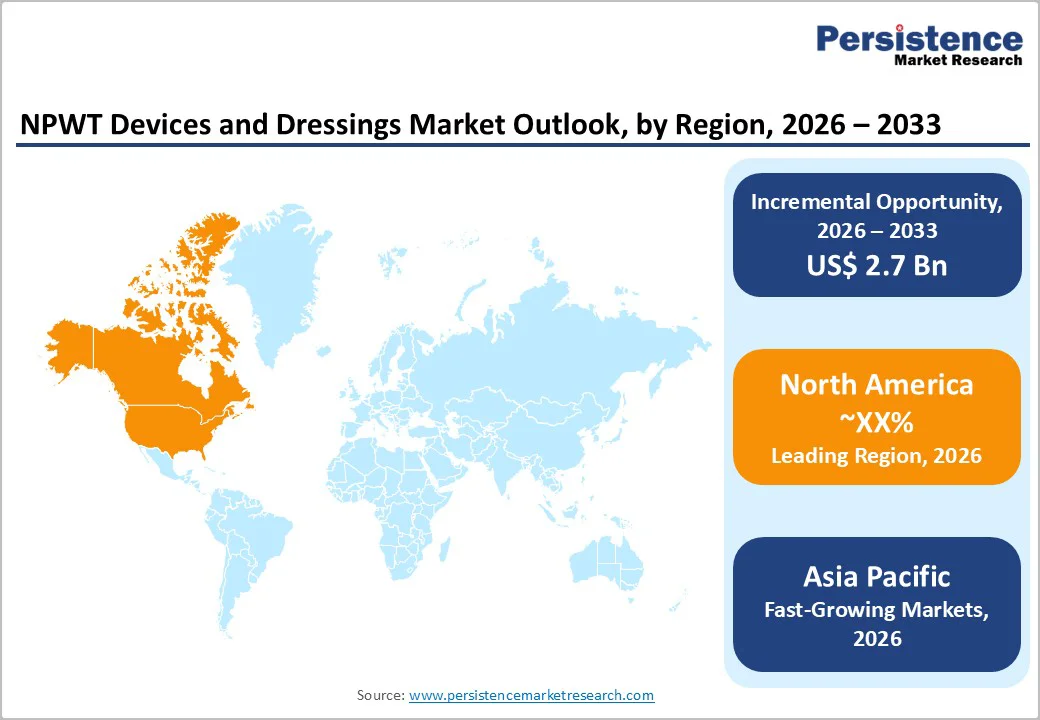
North America leads the NPWT Devices and Dressings market due to rapid technology integration, strong reimbursement coverage, and high prevalence of chronic and post-surgical wounds. Hospitals increasingly prefer disposable and portable NPWT systems, enhancing patient mobility and facilitating early discharge. Advanced dressings with antimicrobial layers and leak-prevention seals are witnessing higher adoption, particularly in surgical incision management. In the U.S., demand is further fueled by rising diabetic foot ulcer treatment volumes, expanding home health agencies, and bundled reimbursement for wound care procedures. U.S. clinicians are also adopting digital monitoring NPWT platforms that track pressure stability and exudate trends, improving continuity of care. Additionally, standardized hospital purchasing pathways, structured wound-care coding systems, and outcome-based reimbursement models make the U.S. the most commercially attractive market within North America.
Asia Pacific NPWT Devices and Dressings market is expanding rapidly, driven by rising diabetes burden, increasing plastic and reconstructive surgeries, and adoption of advanced wound management in tertiary hospitals. Demand is shifting from conventional large NPWT units to lightweight, portable systems suitable for prolonged home use. Governments in India, China, and Southeast Asia are expanding reimbursement coverage for chronic wound treatment and encouraging the use of dressing kits. Local manufacturing of foam dressings and incisional sealant kits is reducing procurement costs and enabling wider hospital-level use. Additionally, clinical training programs for wound clinicians are improving protocol-based NPWT application. Growing medical tourism in Thailand, South Korea, and Malaysia is also boosting the adoption of advanced dressings for post-operative recovery pathways.
The NPWT devices and dressings market is moderately consolidated, with a strong presence of established global manufacturers competing through product innovation, cost optimization, and expanded clinical applications. Competition is shifting toward portable, lightweight, and digitally monitored NPWT units that support home-care usage. Dressing manufacturers are differentiating through antimicrobial coatings, improved exudate handling, and leak-prevention seals. Strategic activities include partnerships with hospitals, rental-based models, and subscription supply of dressing kits.
The global NPWT devices and dressings market size is projected to be valued at US$3.8 Bn in 2026.
Increasing cases of diabetic foot ulcers, venous leg ulcers, pressure injuries, and post-surgical wounds are fueling demand.
The global NPWT devices and dressings market is poised to witness a CAGR of 6.4% between 2026 and 2033.
Integration of micro-sensors to monitor exudate levels, leakage, and pressure, enabling remote patient monitoring.
Avery Dennison Corporation, Cardinal Health, Smith & Nephew, Inc., Medela, and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author