H1N1 Vaccine Market Size and Forecast Analysis
Market Overview
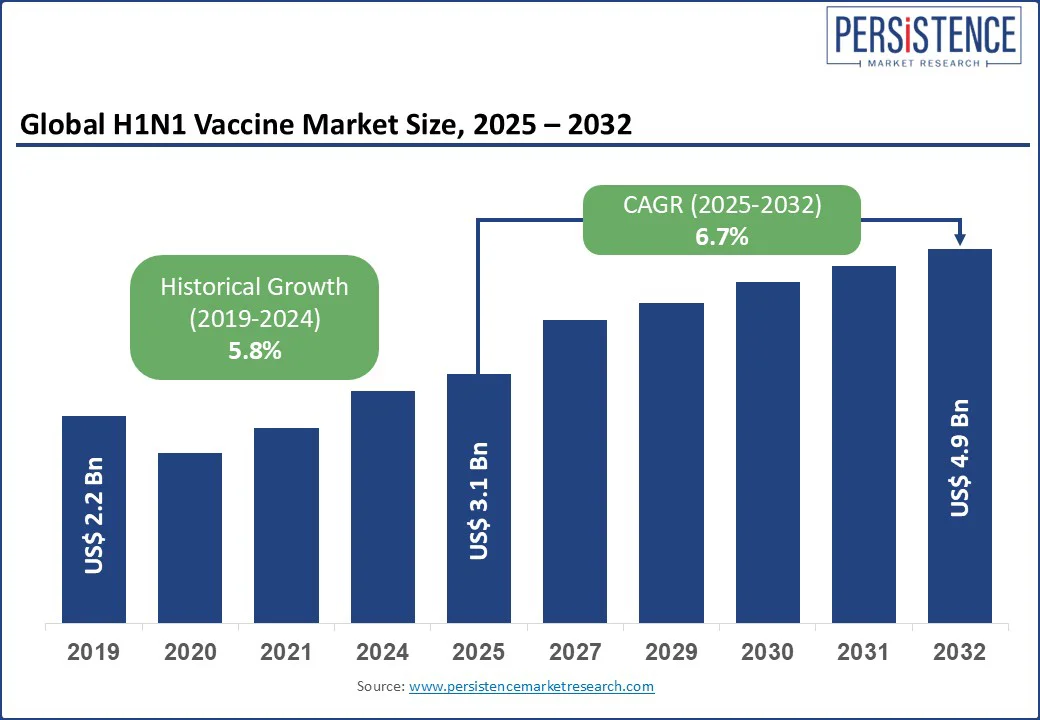
The global H1N1 vaccine market size is likely to be valued at US$3.1 Bn in 2025, and is projected to grow to US$4.9 Bn by 2032, achieving a CAGR of 6.7% from 2025 to 2032. This growth reflects increasing demand for effective influenza prevention, advancements in recombinant vaccine technology, and rising government initiatives to combat seasonal and pandemic influenza outbreaks caused by the H1N1 virus.
The market's growth is underpinned by global health organizations' emphasis on influenza surveillance and preparedness for outbreaks, coupled with innovations in influenza vaccine production and delivery. Key players, including Abbott Laboratories, AstraZeneca plc, Sanofi (Sanofi Pasteur), and GlaxoSmithKline (GSK), dominate the H1N1 Vaccine market, leveraging their expertise in H1N1 immunization shot development and global distribution networks to meet rising demand for the pandemic flu vaccine.
Market Dynamics
Drivers
- Rising H1N1 Incidence Fuels Vaccine Market Demand: The persistent circulation of H1N1 virus strains, as part of seasonal influenza, robustly fuels demand in the H1N1 vaccine market. According to the World Health Organization (WHO), seasonal influenza—including Influenza A vaccine targets such as H1N1—results in 3 to 5 million severe cases and 290,000 to 650,000 respiratory deaths globally every year. The H1N1 virus remains a dominant subtype driving these figures. This prevalence strengthens uptake of the swine flu shot in regions with repeated seasonal outbreaks, particularly North America and Europe, where high-risk groups including the elderly, young children, pregnant individuals, and those with chronic conditions receive strong public health messaging about influenza prevention and access to the best H1N1 vaccine for children and adults.
The U.S. Centers for Disease Control and Prevention (CDC) reported that during the 2024–2025 flu season, influenza activity reached "high severity," with over 44 million illnesses, 580,000 hospitalizations, and more than 25,000 deaths. The CDC continues to make strong recommendations for annual flu vaccine administration for everyone aged six months and older, including queries on where to get the H1N1 vaccine in the US.
- Increasing Awareness and Vaccination Coverage Boost H1N1 Vaccine Market: Growing public awareness and expanding vaccination coverage are key drivers of the H1N1 vaccine market. Public health campaigns by organizations such as the CDC and WHO have significantly increased understanding of influenza risks and H1N1 influenza vaccine side effects, emphasizing the importance of the swine flu shot. These campaigns target high-risk groups, such as children, pregnant women, and the elderly, driving demand for seasonal flu vaccines.
For instance, initiatives such as the CDC’s National Influenza Vaccination Week have boosted uptake by promoting accessibility through pharmacies and clinics, addressing queries such as where to get the H1N1 vaccine in the US. In Europe, the European Centre for Disease Prevention and Control (ECDC) reported increased vaccination rates in countries such as the UK, where H1N1 vaccines are prioritized. Government-backed programs, such as Canada’s FluWatch+, highlight the H1N1 virus dominance in seasonal outbreaks, further encouraging influenza prevention.
Enhanced awareness, supported by trusted health authorities and accessible vaccination sites, continues to fuel market growth by ensuring consistent demand for pandemic flu vaccines across diverse populations.
Restraint
- High Development and Production Costs: Developing influenza vaccines requires significant investment in research, clinical trials, and manufacturing. For instance, developing a new H1N1 immunization shot can cost upwards of US$1 billion, limiting smaller players’ market entry and affecting affordability, despite efforts to mitigate H1N1 influenza vaccine side effects. These high costs reduce market competition, and contribute to pricing challenges despite ongoing efforts to improve safety and minimize side effects.
Opportunities
- Advancements in Delivery Systems: Innovations in intranasal and needle-free delivery systems for H1N1 vaccines could enhance patient compliance and market penetration, particularly for pediatric and elderly populations seeking the best H1N1 vaccine for children and convenient flu vaccine options. For instance, AstraZeneca’s FluMist, an intranasal vaccine, saw a 20% uptake increase in U.S. schools in 2024, per CDC data.
- Combination Vaccine and Seasonality Synergies: Combination COVID-flu vaccines, such as Moderna’s mRNA-1083 targeting H1N1, simplify seasonal immunization by reducing the number of injections. This enhances patient compliance and clinic efficiency during peak vaccination periods in spring and fall. According to the CDC, co-administered vaccines increase uptake, especially among adults over 60. In 2023, the U.S. allocated over $1.5 billion for combo vaccine development, reflecting strong governmental support for integrated immunization strategies.
Category-wise Insights
Product Type Insights
- Inactivated vaccines dominate the H1N1 vaccine market, accounting for approximately 87% of the revenue share in 2025. Their widespread use in seasonal flu vaccine programs, high efficacy, and established safety profile drive their dominance, minimizing concerns about H1N1 influenza vaccine side effects. Their established manufacturing processes, utilized by companies such as Sanofi and GSK, ensure scalability and cost-effectiveness, with global production exceeding 500 million doses annually, per WHO.
- Live attenuated vaccines are the fastest-growing, driven by their ease of administration (intranasal) and suitability for pediatric populations, making them a preferred choice for the best H1N1 vaccine for children. Increasing adoption in school-based vaccination programs, particularly in North America and Europe, and their suitability for mass immunization campaigns in emerging markets contribute to rapid market expansion.
Route of Administration Insights
- Intramuscular vaccines hold the largest market share, around 66% in 2025, due to their widespread use in adult immunization programs and compatibility with inactivated vaccines for influenza prevention. This route ensures high immunogenicity and is standard in hospital and clinic settings, with over 400 million doses administered globally in 2024, per WHO.
- Intranasal vaccines are the fastest-growing, driven by their non-invasive nature and increasing adoption in pediatric and school-based H1N1 vaccine programs. Expanding use in public health campaigns in Asia Pacific, where needle-free options are preferred, and innovations in delivery devices further accelerate growth.
Distribution Channel Insights
- The public distribution channel accounts for over 70% of the H1N1 vaccine market share in 2025, driven by government-led influenza surveillance and immunization programs, including bulk procurement for pandemic flu vaccines. Public channels ensure equitable access, particularly in low-income regions, supported by WHO and GAVI initiatives, making them the primary distribution mode globally.
The private distribution channel is the fastest-growing, fueled by rising demand for flu vaccines in private healthcare facilities and pharmacies in developed markets. Partnerships with retail pharmacies are expanding private sector reach.
Regional Insights
North America H1N1 Vaccine Market Trends
North America holds the largest H1N1 vaccine market share, approximately 41% in 2025, with the U.S. contributing significantly due to its robust healthcare infrastructure and high vaccination coverage.
- Technological advancements, such as recombinant vaccine technology for cell-based vaccines, and the strong presence of key players such as Abbott and GSK, further bolster growth, with widespread availability addressing where to get the H1N1 vaccine in the US.
- Robust Immunization Programs: The U.S. dominates the major regional share, driven by CDC recommendations for universal flu vaccination, with 174 million doses administered in 2024.
Europe H1N1 Vaccine Market Trends
Germany, the UK, and France lead the European H1N1 vaccine market. Germany’s advanced healthcare system and high vaccination rates drive demand for seasonal flu vaccines.
- U.K.: The UK’s National Health Service (NHS) immunization programs and France’s focus on pediatric vaccination, including the best H1N1 vaccine for children, contribute to growth.
- Germany: Germany’s mandatory flu shot recommendations for the elderly and healthcare workers, with 20 million doses distributed in 2024, fuel demand, per the Robert Koch Institute.
- France: France’s focus on pediatric vaccination, with 5 million children vaccinated in 2024, and R&D investments by Sanofi drive market growth.
The region’s market is supported by ECDC influenza surveillance initiatives and regulatory focus on H1N1 influenza vaccine side effects and safety.
Asia Pacific H1N1 Vaccine Market Trends
India and China dominate the Asia Pacific H1N1 vaccine market, driven by large population and increasing healthcare investments.
- India: India’s Universal Immunization Programme, targeting 27 million children annually, includes H1N1 immunization shots, while China’s growing middle class and government support for influenza prevention fuel demand for pandemic flu vaccines. Influenza surveillance in these countries further supports market growth.
- China: China’s market grows with government initiatives targeting 50% flu vaccination coverage by 2030, with 60 million doses distributed in 2024, per NHC data.
Japan: Japan’s advanced healthcare system and high elderly vaccination rates (80% in 2024, per MHLW) support market expansion, led by Novartis.
Competitive Landscape
The global H1N1 vaccine market is highly competitive. Companies such as Sanofi and GSK invest heavily in next-generation influenza vaccines, focusing on recombinant vaccine technology and universal vaccines. Collaborations with governments and organizations such as the WHO enhance market access for pandemic flu vaccines, particularly in developing regions. Players such as Serum Institute and Bharat Biotech focus on affordable H1N1 vaccines to capture emerging markets, addressing accessibility concerns such as where to get the H1N1 vaccine in the US and globally.
Key Developments
- Sanofi (2024): Launched a high-dose H1N1 vaccine for the elderly, improving efficacy in high-risk populations while addressing H1N1 influenza vaccine side effects.
- GSK (2023): Partnered with the WHO to supply swine flu shots to low-income countries, expanding its global footprint.
- Serum Institute of India (2024): Developed a low-cost intranasal H1N1 immunization shot, targeting emerging markets with a focus on influenza prevention.
Companies Covered in H1N1 Vaccines Market
- Abbott Laboratories
- AstraZeneca plc
- Sanofi (Sanofi Pasteur)
- GlaxoSmithKline (GSK)
- CSL Limited
- Novartis
- Bharat Biotech
- Sinovac
- Serum Institute of India
- Zoetis Inc.,
- CPL Biologicals Pvt. Ltd.
Frequently Asked Questions
The H1N1 vaccine market is projected to reach US$3.1 billion in 2025.
High H1N1 virus incidence, increasing vaccination coverage, government initiatives, and advancements in recombinant vaccine technology are the key market drivers.
The H1N1 Vaccine market is poised to witness a CAGR of 6.7% from 2025 to 2032.
Advancements in delivery systems and the development of universal influenza vaccines are the key market opportunities.
Abbott Laboratories, AstraZeneca plc, Sanofi (Sanofi Pasteur), GlaxoSmithKline (GSK), CSL Limited, Novartis, Bharat Biotech, Sinovac, Serum Institute of India, Zoetis Inc., and CPL Biologicals Pvt. Ltd. are among the key players in the global vaccine market.
Global H1N1 Vaccine Market Report Scope
|
Report Attribute
|
Details
|
|
Historical Data/Actuals
|
2019-2024
|
|
Forecast Period
|
2025-2032
|
|
Market Analysis
|
Value: US$ Bn/Mn, Volume: As Applicable
|
|
2025 (E)
|
US$ 3.1 Bn
|
|
2032 (F)
|
US$ 4.9 Bn
|
|
Historical CAGR (2019-2024)
|
5.8%
|
|
Projected CAGR (2025-2032)
|
6.7%
|
|
Geographical Coverage
|
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
|
|
Segmental Coverage
|
- Product Type
- Route of Administration
- Distribution Channel
- Region
|
|
Competitive Analysis
|
- Abbott Laboratories
- AstraZeneca plc
- Sanofi (Sanofi Pasteur)
- GlaxoSmithKline (GSK)
- CSL Limited
- Novartis
- Bharat Biotech
- Sinovac
- Serum Institute of India
- Zoetis Inc.,
- CPL Biologicals Pvt. Ltd.
- Others.
|
|
Report Highlights
|
- Market Forecast and Trends
- Competitive Intelligence and Share Analysis
- Growth Factors and Challenges
- Strategic Growth Initiatives
- Pricing Analysis
- Future Opportunities and Revenue Pockets
- Market Analysis Tools
|
|
Customization and Pricing
|
Available upon request
|
Market Segmentation
By Product Type
- Inactivated Vaccine
- Live Attenuated Vaccine
Route of Administration
- Intradermal
- Intranasal
- Intramuscular
Distribution Channel
By Region
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East and Africa

