ID: PMRREP34132| 223 Pages | 27 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

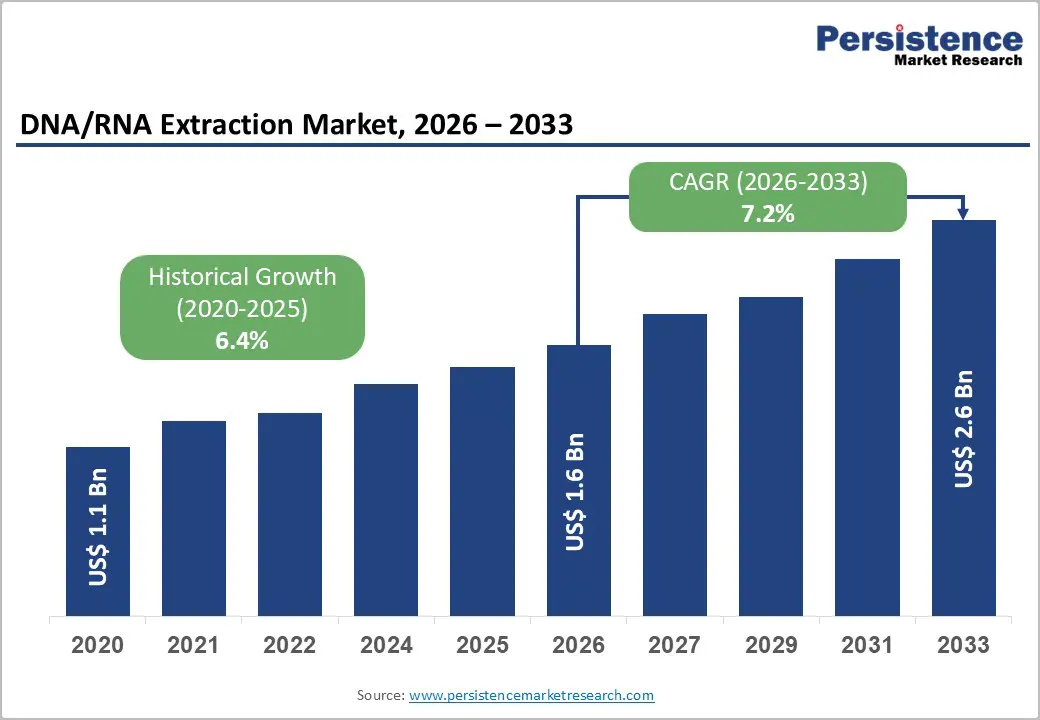
The global DNA/RNA extraction market size is likely to be valued at US$1.6 billion in 2026, and is expected to reach US$2.6 billion by 2033, growing at a CAGR of 7.2% during the forecast period from 2026 to 2033, driven by the increasing prevalence of molecular diagnostics and precision oncology, rising demand for high-throughput nucleic acid purification in research and clinical settings, and advancements in automated magnetic bead-based and column-based extraction technologies. Growing demand for reliable, scalable DNA/RNA extraction kits and instruments, especially viral RNA isolation kits post-pandemic, is accelerating adoption across applications. Advances in reagent-free and rapid extraction methods are further boosting uptake by offering faster turnaround and higher purity. Increasing recognition of DNA/RNA extraction as critical for NGS, PCR diagnostics, and biopharma development in emerging precision medicine markets remains a major driver of market growth.
| Key Insights | Details |
|---|---|
| DNA/RNA Extraction Market Size (2026E) | US$1.6 Bn |
| Market Value Forecast (2033F) | US$2.6 Bn |
| Projected Growth (CAGR 2026 to 2033) | 7.2% |
| Historical Market Growth (CAGR 2020 to 2025) | 6.4% |
The rising demand for molecular diagnostics is a major growth driver for the DNA/RNA extraction market, as accurate and rapid detection of genetic material has become central to modern healthcare. Hospitals and diagnostic laboratories are increasingly relying on PCR, RT-PCR, and next-generation sequencing to identify infectious diseases, genetic disorders, and cancer biomarkers at an early stage. These techniques require highly pure and consistent nucleic acid samples, which is accelerating the adoption of advanced extraction machines and optimized purification kits.
Laboratories are under pressure to process a growing volume of samples within shorter turnaround times, especially for large-scale screening and population-level testing programs. This has fueled a strong demand for high-throughput nucleic acid purification systems that can handle dozens to hundreds of samples in a single run with minimal manual intervention. Automated magnetic bead and column-based platforms reduce human errors, improve reproducibility, and ensure standardized results across batches, making them essential for clinical and research workflows.
The complexity of workflows and the need for skilled personnel are significant restraints in the DNA and RNA extraction market, particularly as laboratories adopt more advanced and automated technologies. Nucleic acid extraction is not a single-step process; it involves multiple stages such as sample preparation, lysis, binding, washing, and elution, each of which must be carefully optimized to avoid degradation or contamination. Even with automated platforms, operators must understand protocol selection, reagent handling, and instrument calibration to ensure consistent and reliable results.
Many laboratories struggle with a shortage of trained molecular biology professionals who can confidently operate high-throughput extraction systems and troubleshoot technical issues. Improper handling or incorrect protocol execution can lead to low yields, poor purity, or failed downstream PCR and sequencing assays, increasing repeat testing and operational costs. Integrating extraction systems into existing laboratory information systems and downstream diagnostic workflows requires technical expertise in both molecular science and laboratory automation. Continuous training is also necessary as manufacturers frequently update software, chemistries, and hardware platforms.
The increasing adoption of next-generation sequencing (NGS) and liquid biopsy technologies is creating a powerful growth opportunity for the DNA and RNA extraction market. NGS has become a core tool in genomics, oncology, and infectious disease research as it enables rapid, high-resolution analysis of entire genomes, exomes, and transcriptomes. The accuracy of sequencing results depends heavily on the quality of extracted nucleic acids. This is driving a strong demand for advanced extraction kits and automated systems that can deliver high-purity, high-yield DNA and RNA from diverse and often low-input samples such as blood, tissue biopsies, and formalin-fixed paraffin-embedded (FFPE) specimens.
Liquid biopsy, which examines circulating tumor DNA (ctDNA), cell-free DNA (cfDNA), and RNA in blood, is driving increasing demand for advanced molecular testing. These biomarkers are present in extremely low quantities and are highly fragmented, making their extraction particularly challenging. To ensure accurate detection, laboratories need highly sensitive purification methods that minimize loss and contamination while maintaining consistency. As next-generation sequencing (NGS) and liquid biopsy transition from research settings to routine clinical practice, laboratories are scaling up testing volumes and implementing standardized, high-throughput extraction workflows. According to the European Society of Medicine, plasma-based NGS for genomic profiling in non-small cell lung cancer (NSCLC) identifies actionable mutations in approximately 48% of patients, with concordance rates of nearly 98% when compared to traditional tissue genotyping.
Kits & reagents are expected to dominate the market, accounting for approximately 60% of the market share in 2026. Its dominance is driven by recurring consumable nature, high-throughput compatibility, and customization for specific sample types, making it preferred for routine extraction. Kits & reagents provide standardized performance, ensure reproducibility, and contribute to workflow efficiency, making it suitable for large-scale diagnostic campaigns. Qiagen’s QIAamp and RNeasy kit lines are widely used across clinical, research, and diagnostics laboratories as they provide high-purity nucleic acids, consistent performance, and compatibility with both manual and automated high-throughput systems. These kits support diverse sample types (blood, tissue, swabs, etc.) and are integrated into workflows for PCR, sequencing, and genomic applications.
Instruments are expected to be the fastest-growing segment, as laboratories increasingly adopt automation to meet the demand for higher throughput, accuracy, and consistency. Automated extraction platforms reduce manual handling, minimize errors, and integrate seamlessly with downstream workflows such as PCR and sequencing. This growth is fueled by large diagnostic laboratories, genomics centers, and public health programs investing in scalable systems capable of processing large numbers of samples quickly, thereby improving turnaround times and operational efficiency in both routine and high-volume testing. The Thermo Scientific KingFisher Apex exemplifies the forefront of automated purification for DNA, RNA, proteins, and cells. Building on decades of the KingFisher product legacy, it combines advanced instrument capabilities with intuitive touchscreen control, offering exceptional flexibility and performance. The KingFisher Apex streamlines sample preparation, making the process faster and more reliable.
Magnetic bead-based methods are expected to currently lead the market, capturing around 40% of the share in 2026. Their popularity is driven by compatibility with automation, scalability, and high recovery rates, making them the preferred choice for clinical workflows. Their dominance is expected to continue as laboratories increasingly expand throughput. The rising adoption of manual column-based methods and expanded reagent-based approaches underscores the growing interest in cost-effective alternatives. For example, New England Biolabs launched the Monarch Mag Viral DNA/RNA Extraction Kit, a magnetic bead-based solution designed for efficient viral nucleic acid recovery and high-throughput applications, demonstrating how these products are tailored for diverse clinical sample types.
Reagent-based extraction is likely to be the fastest-growing segment, fueled by demand for low-cost solutions and expanding use in point-of-care settings. The trend toward simpler, more accessible platforms is accelerating adoption. Innovations in lysis buffers and the development of one-tube extraction methods entering field trials are further driving growth. QuickExtract™ DNA Extraction Solution, for instance, can process anywhere from a single sample to hundreds in just three to eight minutes using only reagent chemistry, making it a cost-effective option for PCR-based applications such as genotyping and viral screening. Its scalability and ease of use make it particularly attractive for research laboratories and point-of-care environments, highlighting how reagent-based solutions are broadening accessibility and driving market adoption beyond traditional instrument-dependent extraction methods.
Oncology is projected to lead the market, accounting for nearly 35% of revenue in 2026. This dominance is driven by its continued role as the primary application for tumor profiling, large-scale liquid biopsy programs, and the management of diverse samples requiring high-quality nucleic acids. The sector benefits from strong integration, skilled pathologists, and the capability to handle both high-volume and low-input samples, which drives higher reagent and instrument consumption. Oncology applications are also spearheading the adoption of magnetic bead technologies and conducting emerging low-input trials. For example, Thermo Fisher Scientific offers the MagMAX™ Cell-Free DNA Isolation Kits, which efficiently recover cfDNA from liquid biopsy samples for downstream cancer analyses. These reagent and instrument combinations are widely utilized in oncology research centers and clinical laboratories, supporting precision medicine initiatives and monitoring treatment responses with high reliability.
The drug discovery and development segment is projected to be the fastest-growing, driven by a strong presence in screening and an expanding role in target validation. It provides convenient, rapid, and accessible purification solutions, appealing to biopharma companies that favor high-throughput, reproducible workflows. Greater outreach initiatives, a focus on screening, and the wider availability of both routine and premium kits are further accelerating adoption, supporting rapid integration across discovery and development stages. QIAGEN N.V. offers a comprehensive portfolio of DNA and RNA extraction kits designed for R&D applications, including RNeasy® and QIAprep® products, which enable fast, consistent purification of nucleic acids from a variety of sample types, crucial for high-throughput screening and validation in drug discovery projects.
Biopharmaceutical companies are projected to lead the market in 2026, capturing roughly 30% of the share, driven by the high demand for genomic workflows and the strong global focus on cell and gene therapy development. Consistent demand is supported by regular R&D schedules, sample processing needs, and broad access to automated instruments. Increased emphasis on academic laboratories and diagnostic centers further reinforces the dominant position of biopharma. Thermo Fisher Scientific Inc. provides widely used MagMAX™ DNA/RNA purification kits, which are paired with KingFisher™ automated extraction systems and extensively adopted by biopharmaceutical companies and large research institutions. These kits are designed for automated workflows, enabling the efficient processing of dozens to hundreds of samples, and support applications in genomics, transcriptomics, and biomarker discovery, key areas in drug development and cell and gene therapy research.
Academic and research laboratories are likely to be the fastest-growing segment, driven by the increasing demand for NGS sample preparation, reliance on grant funding, and broader adoption of multi-omics approaches. Enhanced throughput, specialized kits, and stronger collaboration on novel methodologies are facilitating rapid adoption. The expanding use of hospital laboratories, CROs, and other high-tech sectors is further accelerating market growth. For example, the Genomics Core Facility at the Icahn School of Medicine offers DNA and RNA extraction services as a critical first step for a wide range of downstream applications, including next-generation sequencing (NGS), array processing, and qPCR. The facility supports high-throughput extraction from diverse sample types, such as FFPE tissue, blood, cultured cells, and swabs, providing researchers with high-quality nucleic acids essential for complex genomic studies.

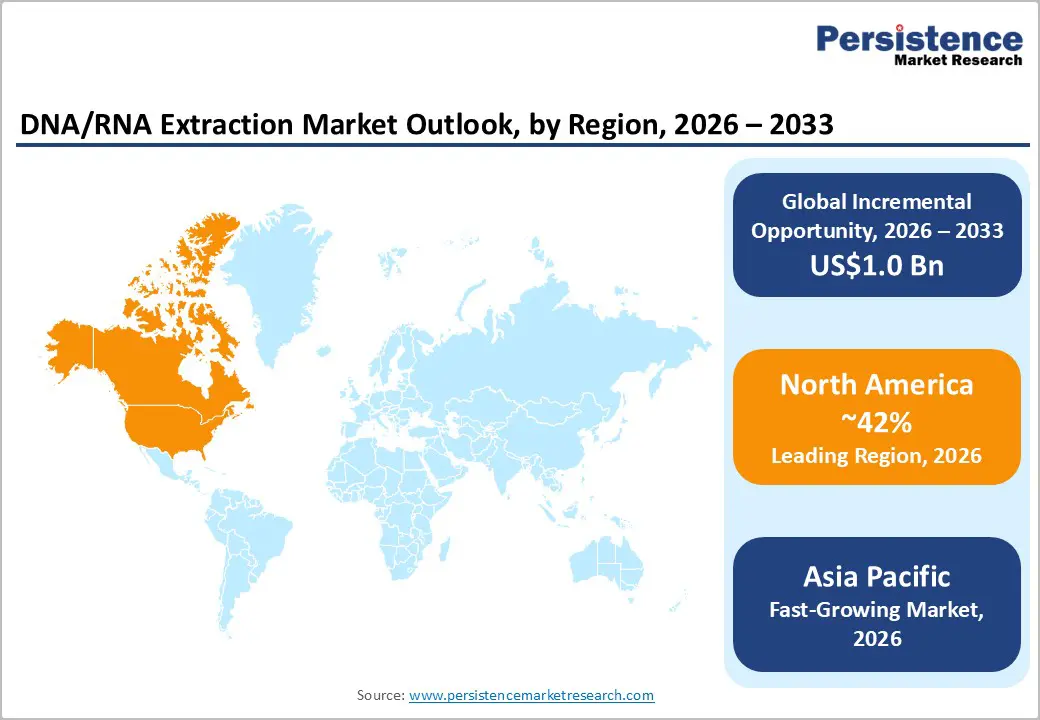
North America is projected to lead the global DNA/RNA extraction market, accounting for nearly 42% of the total market in 2026. This dominance is driven by the region’s advanced biopharmaceutical infrastructure, robust research and development capabilities, and high public awareness of the benefits of precision medicine. Laboratories in the U.S. and Canada provide comprehensive support for extraction programs, ensuring widespread accessibility of DNA/RNA extraction across oncology, drug discovery, and diagnostic applications.
The increasing demand for magnetic bead-based extraction methods, known for their convenience, automation compatibility, and improved throughput, is accelerating adoption, as these technologies reduce the challenges associated with manual extraction techniques. Innovation in DNA/RNA extraction technologies, such as stable low-input protocols, enhanced viral RNA recovery, and targeted next-generation sequencing (NGS) applications, is attracting substantial investment from both public and private sectors. Government initiatives, including NIH campaigns, continue to encourage adoption by addressing sample loss risks, workflow inefficiencies, and emerging genomic threats, thereby sustaining market demand. The growing emphasis on oncology-grade applications and specialty uses, particularly liquid biopsies, is expanding the range of applications for DNA/RNA extraction in the region.
Europe is experiencing steady market growth, driven by increasing awareness of the benefits of molecular technologies, robust healthcare systems, and government-led precision medicine initiatives. Countries such as Germany, France, and the U.K. boast well-established research infrastructures that facilitate routine DNA/RNA extraction and promote the adoption of innovative kit delivery methods. High-quality formulations are especially attractive to oncology patients, regulation-focused laboratories, and drug discovery applications, enhancing yield and coverage rates.
Technological advancements in DNA/RNA extraction, such as improved automation, application-specific delivery systems, and enhanced low-input options, are further expanding market potential. European authorities are actively supporting research and clinical trials for both routine and specialized extraction needs, which is strengthening overall market confidence. The region’s emphasis on convenient, scalable solutions aligns with its focus on preventive diagnostics and reducing sample failure. Public awareness campaigns and promotional efforts are extending reach across both urban and rural areas, while suppliers continue to invest in innovative bead-based technologies and novel variants to improve efficacy.
The Asia Pacific region is poised to become the fastest-growing market for DNA/RNA extraction in 2026, driven by increasing genomics awareness, expanding government initiatives, and broader application programs. Countries such as India, China, Japan, and those in Southeast Asia are actively promoting extraction efforts to support oncology growth and emerging research needs. DNA/RNA extraction is particularly appealing in these regions due to its cost-effectiveness, scalability, and suitability for large-scale laboratory operations in both urban and rural areas.
Technological advancements are enabling the development of stable, efficient, and user-friendly extraction methods that perform well under challenging sample conditions and minimize yield variability. These innovations are essential for reaching remote laboratories and enhancing overall genomic coverage. Rising demand from oncology, drug discovery, and diagnostic applications is further driving market growth. Additionally, public-private partnerships, increased research funding, and growing investment in extraction research and manufacturing capacity are accelerating expansion. The combination of convenient extraction processes, improved purity, and reduced contamination risk is solidifying DNA/RNA extraction as a preferred solution in the region.
The global DNA/RNA extraction market features competition between established life-science leaders and emerging molecular suppliers. In North America and Europe, Thermo Fisher Scientific Inc. and QIAGEN N.V. lead through strong R&D, distribution networks, and clinical ties, bolstered by innovative magnetic bead and automation programs. In Asia Pacific, local firms advance with cost-competitive solutions, enhancing accessibility. Magnetic bead delivery boosts throughput, cuts manual risks, and enables mass integrations across regions. Strategic partnerships, collaborations, and acquisitions merge expertise, expand kits, and speed commercialization. Low-input formulations solve sample issues, aiding penetration in precision areas.
Key Industry Developments
The global DNA/RNA extraction market is projected to reach US$1.6 billion in 2026.
The rising prevalence of molecular diagnostics and precision oncology, and demand for high-throughput nucleic acid purification are key drivers.
The DNA/RNA extraction market is poised to witness a CAGR of 7.2% from 2026 to 2033.
Advancements in automation-compatible and low-input delivery platforms are key opportunities.
Thermo Fisher Scientific Inc., QIAGEN N.V., Promega Corporation, F. Hoffmann-La Roche AG, and Bio-Rad Laboratories, Inc. are the key players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020–2025 |
| Forecast Period | 2026–2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Method
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author