- Executive Summary
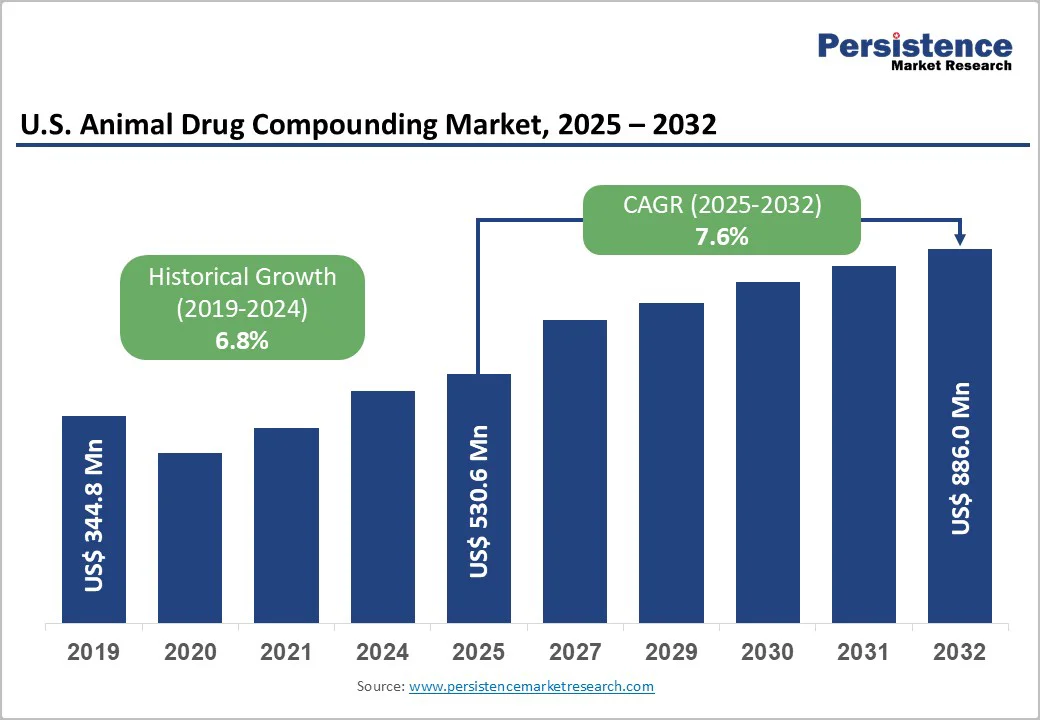
- U.S. Animal Drug Compounding Market Snapshot, 2025 and 2032
- Market Opportunity Assessment, 2025 - 2032, US$ Mn
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments and Key Market Events
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Challenges
- Key Trends
- Macro-Economic Factors
- U.S. Sectorial Outlook
- U.S. GDP Growth Outlook
- COVID-19 Impact Analysis
- Forecast Factors - Relevance and Impact
- Value Added Insights
- Regulatory Landscape
- Key Deals and Mergers
- PESTLE Analysis
- Porter’s Five Force Analysis
- U.S. Animal Drug Compounding Market Outlook:
- Key Highlights
- Market Size (US$ Mn) and Y-o-Y Growth
- Absolute $ Opportunity
- Market Size (US$ Mn) Analysis and Forecast
- Historical Market Size (US$ Mn) Analysis, 2019-2024
- Market Size (US$ Mn) Analysis and Forecast, 2025-2032
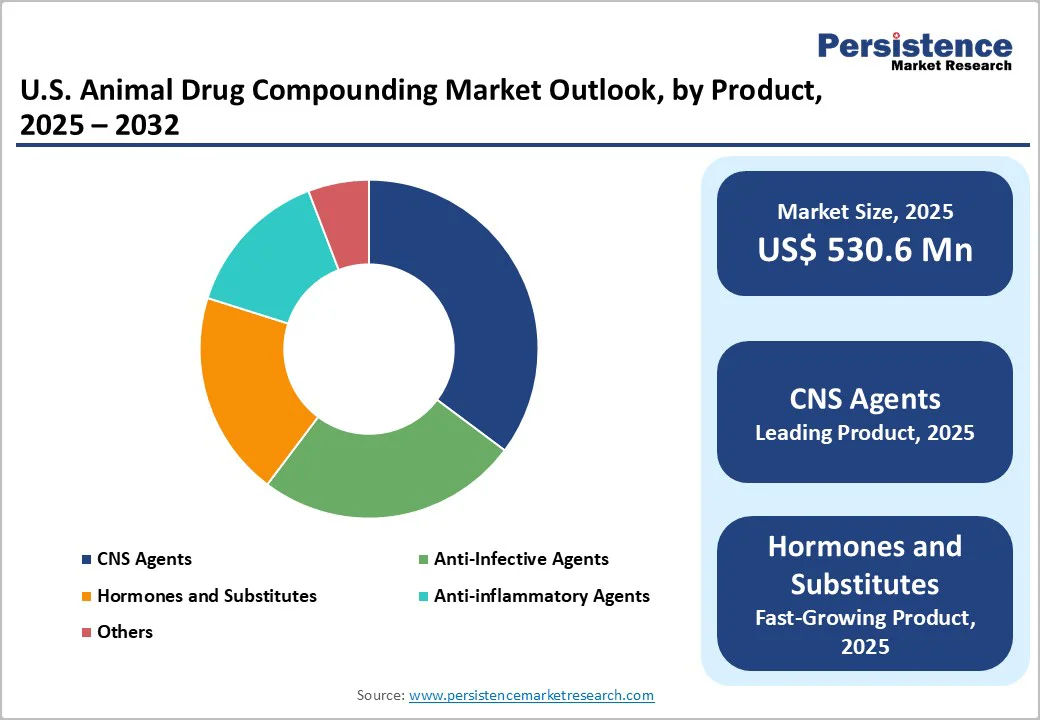
- U.S. Animal Drug Compounding Market Outlook: Product
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Product, 2019 - 2024
- Market Size (US$ Mn) Analysis and Forecast, By Product, 2025 - 2032
- CNS Agents
- Anti-Infective Agents
- Hormones and Substitutes
- Anti-inflammatory Agents
- Others
- Market Attractiveness Analysis: Product
- U.S. Animal Drug Compounding Market Outlook: Animal
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Animal, 2019 - 2024
- Market Size (US$ Mn) Analysis and Forecast, By Animal, 2025 - 2032
- Companion
- Cats
- Dogs
- Others
- Livestock
- Companion
- Market Attractiveness Analysis: Animal
- U.S. Animal Drug Compounding Market Outlook: Formulation
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Formulation, 2019 - 2024
- Market Size (US$ Mn) Analysis and Forecast, By Formulation, 2025 - 2032
- Oral
- Injectable
- Others
- Market Attractiveness Analysis: Formulation
- Key Highlights
- U.S. Animal Drug Compounding Market Outlook: Region
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Region, 2019 - 2024
- Market Size (US$ Mn) Analysis and Forecast, By Region, 2025 - 2032
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- Competition Landscape
- Market Structure
- Competition Intensity Mapping By Market
- Competition Dashboard
- Company Profiles (Details - Overview, Financials, Strategy, Recent Developments)
- Hoye's Pharmacy
- Overview
- Segments and Products
- Key Financials
- Market Developments
- Market Strategy
- Vertisis Custom Pharmacy
- Dougherty's Pharmacy
- Triangle Compounding Pharmacy Inc.
- Medisca Inc.
- Wedgewood Pharmacy
- Millers Pharmacy
- Essential Pharmacy Compounding Veterinary
- Custom Med Compounding Pharmacy, Inc.
- Davis Islands Pharmacy, Inc.
- Wellness Pharmacy of Cary, Inc.
- Cencora, Inc.
- Sixth Avenue Medical Pharmacy
- Bova Compounding
- Others
- Hoye's Pharmacy
- Market Structure
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment