- Executive Summary
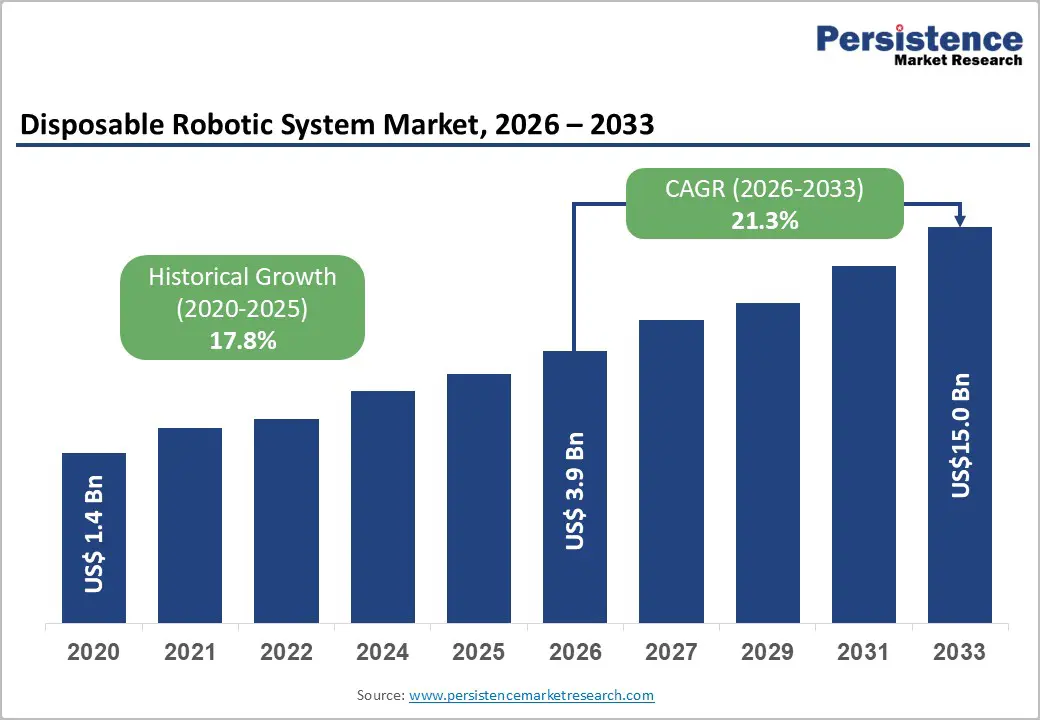
- Global Disposable Robotic System Market Snapshot, 2026 and 2033
- Market Opportunity Assessment, 2026 – 2033, US$ Mn
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments and Key Market Events
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Challenges
- Key Trends
- Product Lifecycle Analysis
- Global Parent Market Overview
- Disposable Robotic System Market: Value Chain
- List of Raw Deployment Mode Supplier
- List of Manufacturers
- List of Distributors
- List of End User Industries
- Profitability Analysis
- Forecast Factors – Relevance and Impact
- Covid-19 Impact Assessment
- PESTLE Analysis
- Porter’s Analysis
- Geopolitical Tensions: Market Impact
- Regulatory and Deployment Mode Landscape
- 3.1. Macro-Economic Factors
- Global Sectorial Outlook
- Global GDP Growth Outlook
- Other Macro-economic Factors
- Price Trend Analysis, 2020 – 2033
- Key Highlights
- Key Factors Impacting Product Prices
- Prices By Product Type/Deployment Mode/End User
- Regional Prices and Product Preferences
- Global Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Market Size and Y-o-Y Growth
- Absolute $ Opportunity
- Market Size (US$ Mn) Analysis and Forecast
- Historical Market Size Analysis, 2020-2025
- Current Market Size Forecast, 2020–2033
- Global Disposable Robotic System Market Outlook: Product Type
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis By Product Type, 2020 – 2025
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Market Attractiveness Analysis: Product Type
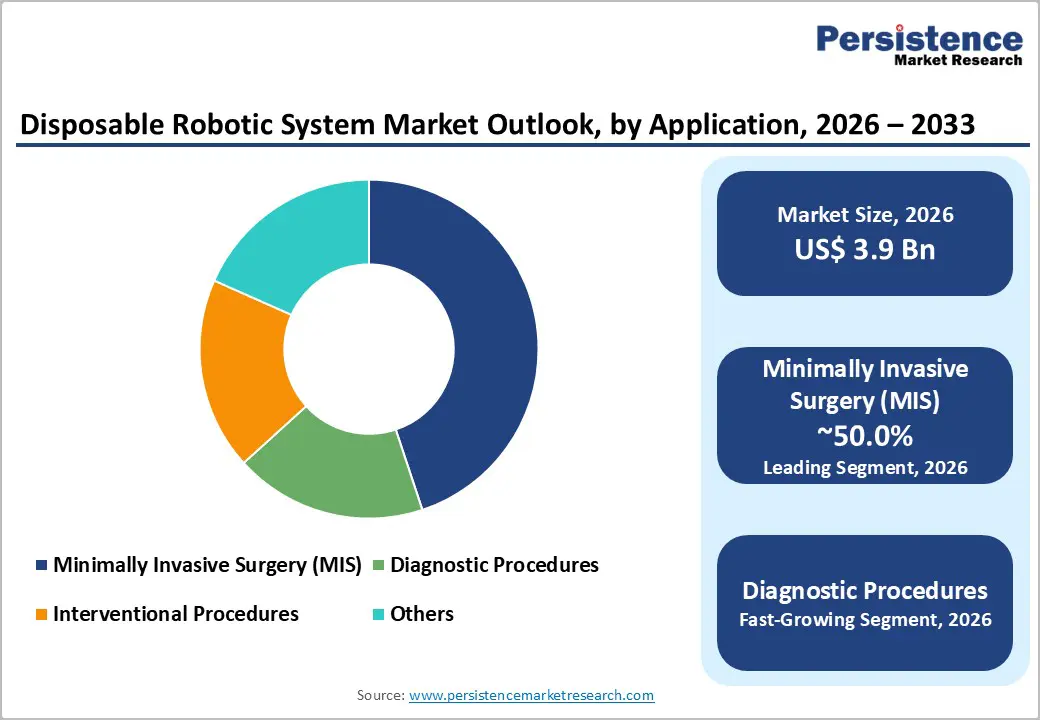
- Global Disposable Robotic System Market Outlook: Application
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis By Application, 2020 – 2025
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Market Attractiveness Analysis: Application
- Global Disposable Robotic System Market Outlook End User
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis By End User, 2020 – 2025
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis: End User
- Key Highlights
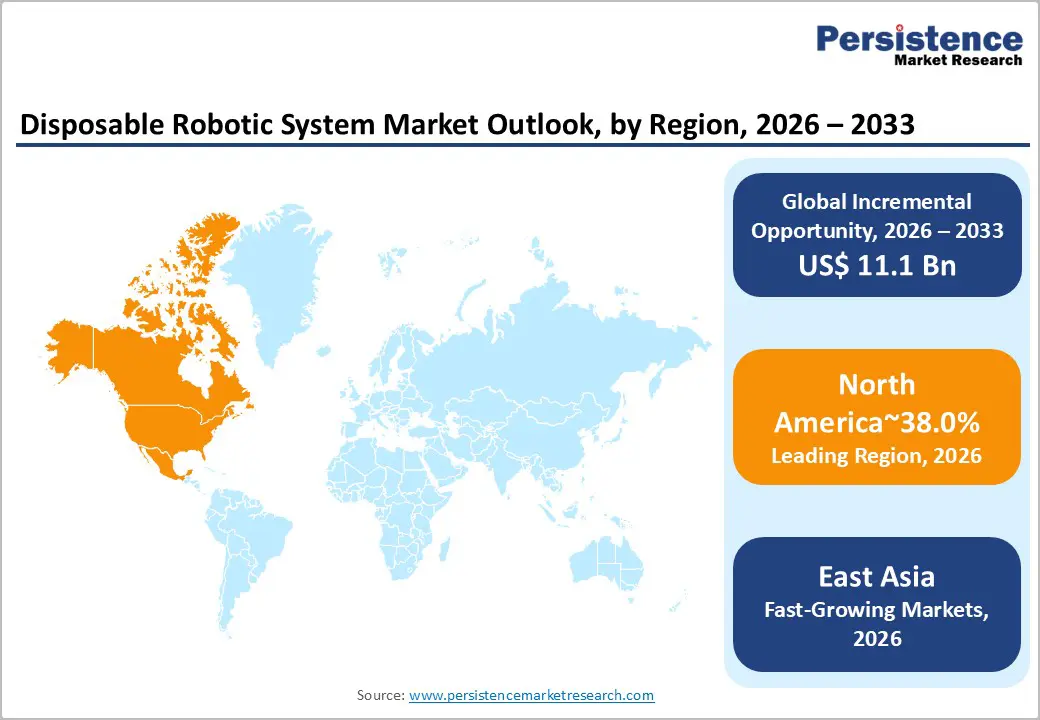
- Global Disposable Robotic System Market Outlook Region
- Key Highlights
- Historical Market Size (US$ Mn) Analysis By Region, 2020 – 2025
- Current Market Size (US$ Mn) Forecast By Region, 2026 – 2033
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- U.S.
- Canada
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- Europe Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- By Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- Germany
- Italy
- France
- U.K.
- Spain
- Russia
- Türkiye
- Rest of Europe
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- East Asia Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- China
- Japan
- South Korea
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- South Asia & Oceania Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- India
- Southeast Asia
- ANZ
- Rest of South Asia & Oceania
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- Latin America Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- Brazil
- Mexico
- Rest of Latin America
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- Middle East & Africa Disposable Robotic System Market Outlook: Historical (2020 – 2025) and Forecast (2026 – 2033)
- Key Highlights
- Pricing Analysis
- Historical Market Size (US$ Mn) Analysis By Market, 2020 – 2025
- By Country
- By Product Type
- By Application
- By End User
- Current Market Size (US$ Mn) Forecast By Country, 2026 – 2033
- GCC Countries
- South Africa
- Northern Africa
- Rest of MEA
- Current Market Size (US$ Mn) Forecast By Product Type, 2026 – 2033
- Disposable Surgical Robots
- Disposable Endoscopic Robotic Systems
- Disposable Catheter-based Robotic Systems
- Others
- Current Market Size (US$ Mn) Forecast By Application, 2026 – 2033
- Minimally Invasive Surgery (MIS)
- Laparoscopy
- Orthopedic Surgery
- Urology
- Gynecology
- Cardiothoracic Surgery
- Diagnostic Procedures
- Interventional Procedures
- Others
- Minimally Invasive Surgery (MIS)
- Current Market Size (US$ Mn) Forecast By End User, 2026 – 2033
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- Others
- Market Attractiveness Analysis
- Competition Landscape
- Market Share Analysis, 2025
- Market Structure
- Competition Intensity Mapping By Market
- Competition Dashboard
- Apparent Production Capacity
- Company Profiles (Details – Overview, Financials, Strategy, Recent Developments)
- Intuitive Surgical
- Overview
- Segments and Products
- Key Financials
- Market Developments
- Market Strategy
- Smith & Nephew
- Microdot Medical
- Medtronic
- EndoWays
- Stryker Corporation
- Zimmer Biomet Holdings
- TransEnterix Surgical
- Verb Surgical
- Medicaroid
- Intuitive Surgical
Note: List of companies is not exhaustive in Nature. It is subject to further augmentation during course of research
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations