ID: PMRREP2827| 201 Pages | 16 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

The global biopharmaceutical market size is valued at US$515.8 billion in 2025 and is projected to reach US$910.7 billion, growing at a CAGR of 8.5% between 2025 and 2032. Biopharmaceuticals are advanced therapeutic products manufactured using biotechnology-based processes such as recombinant DNA engineering, hybridoma technology, and highly controlled purification systems. Derived from living organisms or their cellular components, these products primarily consist of complex proteins and nucleic acids designed to target specific diseases with greater precision than conventional chemical drugs.
| Key Insights | Details |
|---|---|
|
Biopharmaceutical Market Size (2025E) |
US$515.8 Billion |
|
Market Value Forecast (2032F) |
US$910.7 Billion |
|
Projected Growth (CAGR 2025 to 2032) |
8.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.4% |
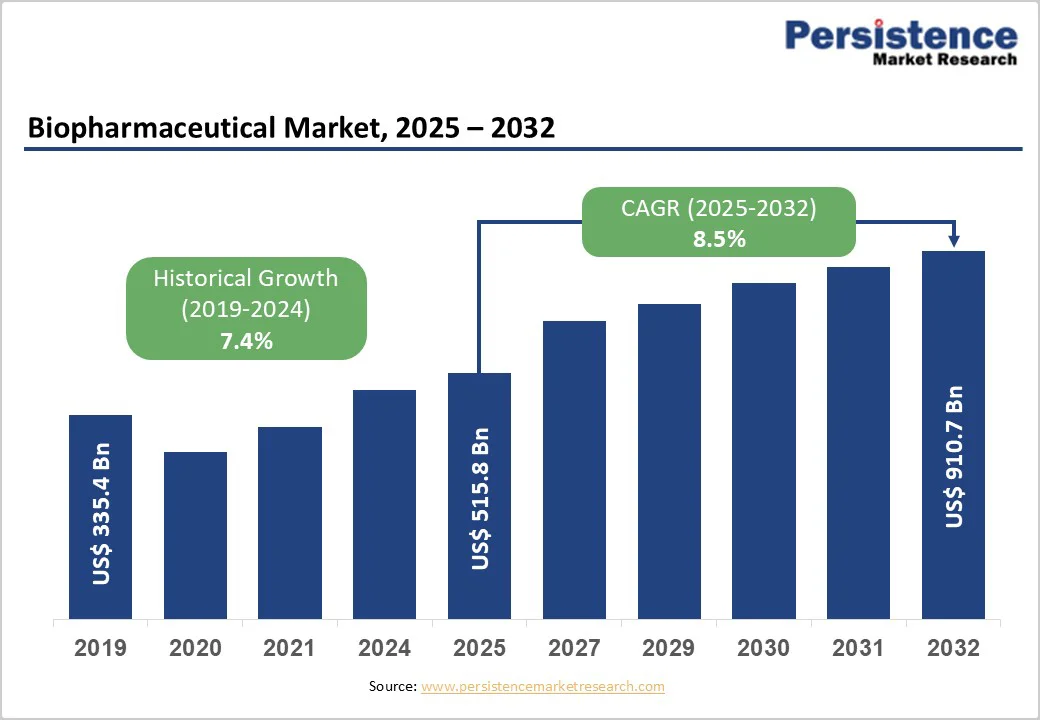
The global biopharmaceutical market is driven by the growing inclination towards targeted therapies and substantial research and development investments, particularly in oncology, neurology, and rare diseases. Protein therapeutics, monoclonal antibodies, erythropoietin, growth hormones, recombinant proteins, recombinant human insulin, interferons, vaccines, and other biopharmaceuticals are witnessing rapid adoption due to their high specificity and improved patient outcomes.
Biopharmaceuticals improve healthcare quality and life expectancy for patients with chronic conditions, including cardiovascular, metabolic, neurological, cancer, and rare diseases. A recent study highlighted that from 2024 to 2025, biologics and small-molecule modalities dominated biopharma research and development dealmaking, with biologics accounting for nearly half of all alliances.
High-value partnerships included RNA, antibody, and immunotherapy deals. At the same time, mergers and acquisitions focused on oncology, neurology, and specialty pharmaceuticals, reflecting a strategic emphasis on both established and emerging therapeutic areas, further underscoring market expansion.
Despite growth, the global biopharmaceutical market faces significant restraints, including high production costs, stringent regulatory requirements, and risks of adverse side effects. Biopharmaceutical manufacturing requires sophisticated infrastructure and adherence to global compliance standards, including U.S. Food and Drug Administration (FDA) regulations and European Medicines Agency (EMA) guidelines, which drive up operational expenditure. The entry of lower-cost biosimilars increases competition, pressuring pricing structures.
Additionally, complex clinical trial protocols and long development timelines pose challenges for small- to mid-sized companies, limiting market access. Fluctuating raw material supply, particularly post-pandemic, adds to production uncertainties. These factors collectively constrain the pace at which biopharmaceutical companies can scale operations and launch new therapeutics globally, while maintaining profitability and regulatory compliance.
The global biopharmaceutical market offers significant opportunities driven by mergers, collaborations, and AI-enabled innovation. In January 2024, the University of Oxford, Barinthus Biotherapeutics, and Coalition for Epidemic Preparedness Innovations (CEPI) partnered to advance a Middle East Respiratory Syndrome (MERS) coronavirus vaccine candidate, VTP-500, with CEPI funding $34.8 million for stockpiling and Phase II trials.
In December 2024, Ikena Oncology and Inmagene Biopharmaceuticals merged to form ImageneBio, which is developing IMG-007, a non-depleting anti-OX40 monoclonal antibody for inflammatory diseases. Further, in September 2024, Sanofi collaborated with OpenAI and Formation Bio to accelerate AI-driven drug discovery, integrating modeling, engineering, and biopharma data expertise.
These strategic collaborations, AI integration, and focus on novel therapeutics enable faster development cycles, enhanced precision, and expanded pipelines, creating lucrative avenues for growth and diversification in the biopharmaceutical industry.
Monoclonal antibodies are projected to capture a dominant 58.3% share of the global biopharmaceutical market by 2025. Their precision targeting, efficacy in oncology and autoimmune disorders, and strong pipeline innovations are driving robust adoption. Increasing approvals, improved safety profiles, and rising prevalence of chronic diseases further boost their preference over other biopharmaceutical products. Market growth is also supported by ongoing research in next-generation antibody therapies, including bispecifics and antibody-drug conjugates, which enhance therapeutic effectiveness and expand clinical applications.
Oncology is expected to dominate the global biopharmaceutical market in 2025 with a 34.3% share. High cancer prevalence, rising awareness, and demand for personalized therapies are key drivers. Innovations in immunotherapies, monoclonal antibodies, and gene-targeted drugs are accelerating adoption. Regulatory approvals increased clinical trials, and improved survival rates contribute to oncology’s leading position. Chronic disease burden and an aging population further enhance market demand, positioning oncology as the primary growth segment. Emerging therapies in hematologic and solid tumors also support sustained market leadership.
Hospital pharmacies are projected to lead the global biopharmaceutical distribution market in 2025 with a 42.6% share. Their advantage stems from direct access to high-value therapies, integration with inpatient treatment protocols, and centralized inventory management. Hospitals handle complex biologics requiring specialized storage and administration, supporting patient adherence and safety. Rising hospital infrastructure, expanded healthcare services, and preference for in-hospital treatment of chronic and critical diseases reinforce this channel. Collaboration with specialty pharmacies and streamlined procurement processes further strengthen hospital pharmacies’ dominance in the global biopharmaceutical distribution network.
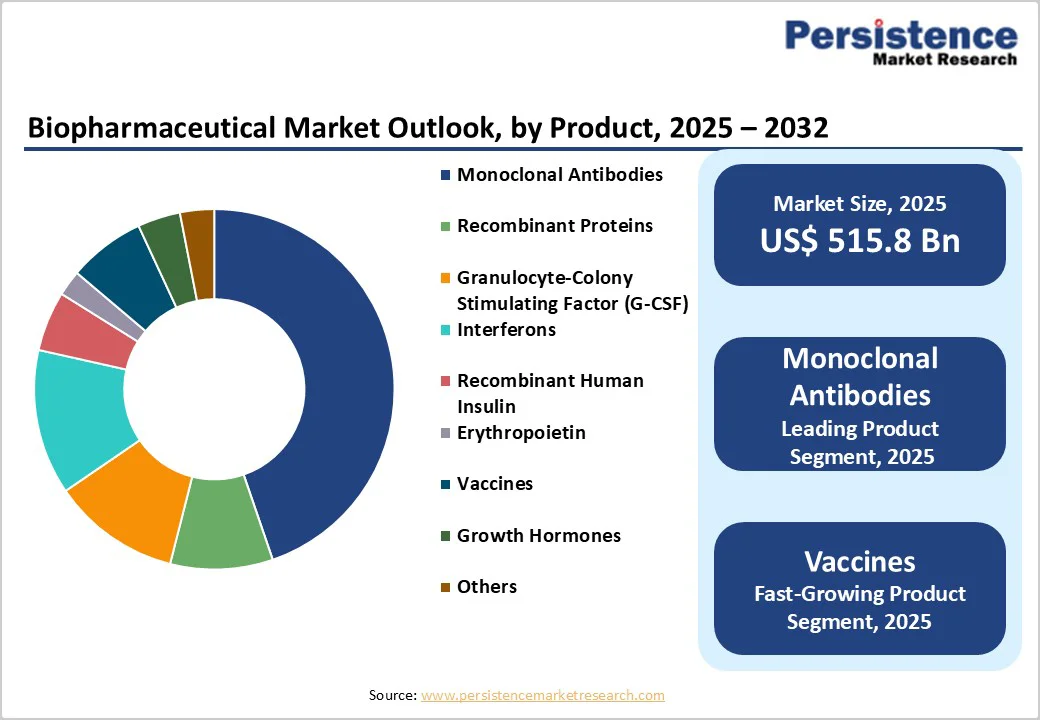
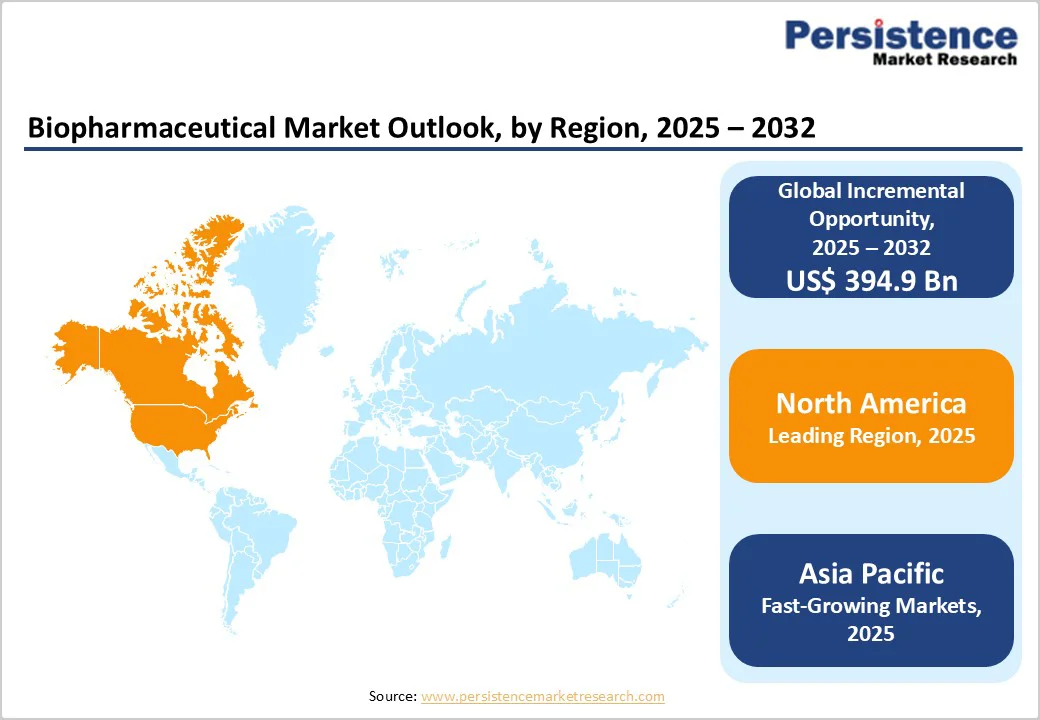
North America is projected to capture nearly 44.8% of the global biopharmaceutical market by 2025, led by technological advancement, oncology-focused therapeutics, and robust outsourcing to contract manufacturing organizations (CMOs).
Demand for monoclonal antibodies is rapidly growing, with wide adoption across chronic and rare diseases. The BIO International Convention, held in San Diego from 3–6 June 2024, brought together over 18,500 industry leaders, underscoring the region’s innovation ecosystem.
In January 2024, the U.S. Food and Drug Administration (FDA) approved CASGEVY™ (exagamglogene autotemcel), the first CRISPR/Cas9-based cell therapy for transfusion-dependent β-thalassemia. In July 2024, Amneal Pharmaceuticals expanded its biosimilar portfolio with an omalizumab (XOLAIR®) candidate.
Accord BioPharma launched Imuldosa® PFS in August 2025, a biosimilar to Stelara® (ustekinumab), following FDA approval in October 2024. In January 2025, Teva Pharmaceutical Industries and Samsung Bioepis entered a U.S. license agreement for EPYSQLI® (eculizumab-aagh), a Soliris® biosimilar, made available at a 30% discount in April 2025. These advancements in approvals, biosimilars, and strategic collaborations reinforce North America’s leadership in biopharmaceuticals.
Europe is projected to hold approximately 27.5% of the global biopharmaceutical market by 2025, driven by regulatory approvals, manufacturing expansion, and strategic licensing. In February 2024, CRISPR Therapeutics’ CASGEVY™ received conditional European Commission approval for the treatment of severe sickle cell disease and transfusion-dependent β-thalassemia, marking the first CRISPR/Cas9 therapy in the EU.
In October 2024, Lonza expanded its biologics manufacturing footprint in Visp, Switzerland, and acquired a large-scale site in Vacaville, USA, to enhance contract development and manufacturing organization (CDMO) capabilities.
In February 2025, Celltrion’s Avtozma® (CT-P47), a biosimilar to RoActemra® (tocilizumab), received European Commission approval for multiple indications. In October 2025, Teva Pharmaceutical Industries signed a license and supply agreement with Prestige Biopharma for Tuznue® (trastuzumab biosimilar), and Eli Lilly’s anti-amyloid therapy donanemab (Kisunla) gained EU approval for early Alzheimer’s disease. These developments in approvals, manufacturing, and strategic partnerships strengthen Europe’s biopharmaceutical growth trajectory.
The Asia Pacific biopharmaceutical market is expected to grow at a CAGR of 10.6%, driven by strategic collaborations, local manufacturing, and regional expansion. The Biopharmaceuticals Alliance, involving India, the United States, South Korea, Japan, and the European Union, was launched during the Bio International Convention 2024 in San Diego to enhance supply chain resilience.
In January 2025, a report highlighted China’s pivot toward Southeast Asia, leveraging real-world data and regional investment to expand influence. In February 2025, Zydus Lifesciences launched VaxiFlu-4, a quadrivalent influenza vaccine, in India.
In June 2025, AstraZeneca partnered with CSPC Pharmaceuticals (China) for AI-enabled preclinical drug discovery targeting immunological diseases. In October 2025, Takara Bio Inc. acquired ViSpot, Inc., integrating viral-safety testing with its CDMO services. These initiatives underscore cross-border collaboration, AI adoption, and enhanced regional manufacturing as key growth drivers for the Asia Pacific market.
The global biopharmaceutical market is highly competitive, driven by innovation-heavy pipelines, strong biosimilar expansion, and aggressive strategic collaborations. Leading players focus on monoclonal antibodies, cell and gene therapies, and next-generation biologics while strengthening manufacturing capacity and global supply chains. Companies increasingly pursue regional partnerships, licensing deals, and acquisitions to expand therapeutic portfolios, accelerate commercialization, and maintain market leadership amid rising pricing pressure and regulatory complexity.
The global biopharmaceutical market is projected to be valued at US$ 515.8 billion in 2025.
Rising demand for targeted biologics, robust R&D investment, and expanding chronic disease burden drive the global market.
The global market is poised to witness a CAGR of 8.5% between 2025 and 2032.
Emerging markets, biosimilars expansion, and advanced platforms such as cell and gene therapies offer major biopharmaceutical growth opportunities.
Major players in the global are Pfizer Inc., AstraZeneca, Sanofi, Bristol-Myers Squibb Company, Biocon, GSK plc, AbbVie Inc., and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Therapeutic Application
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author