ID: PMRREP35651| 189 Pages | 24 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

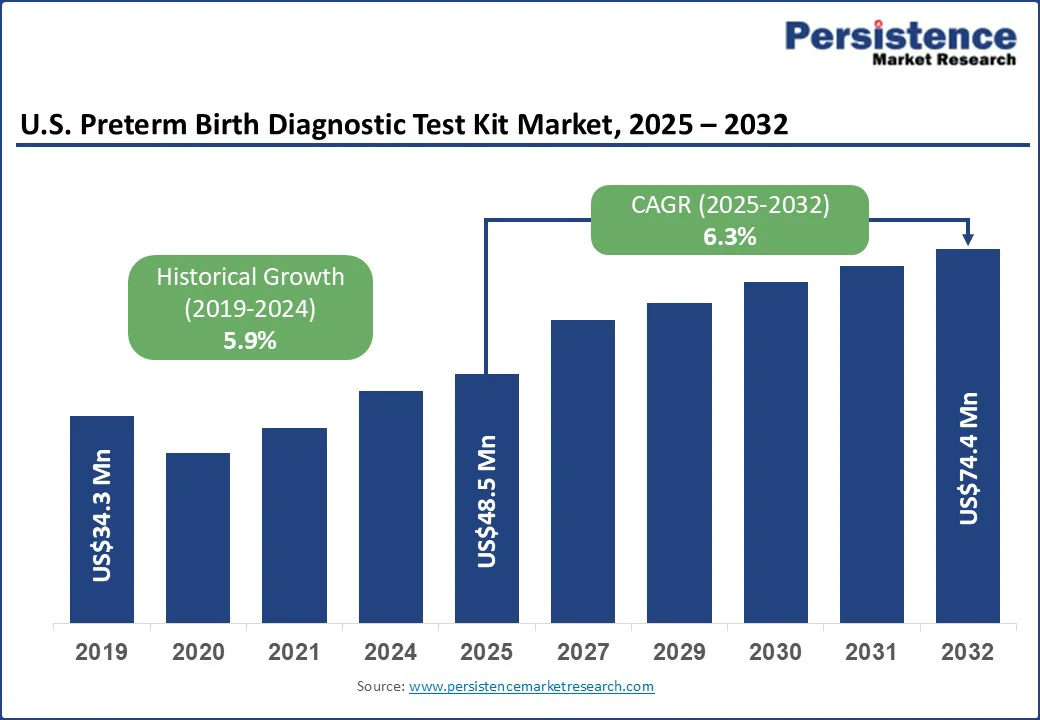
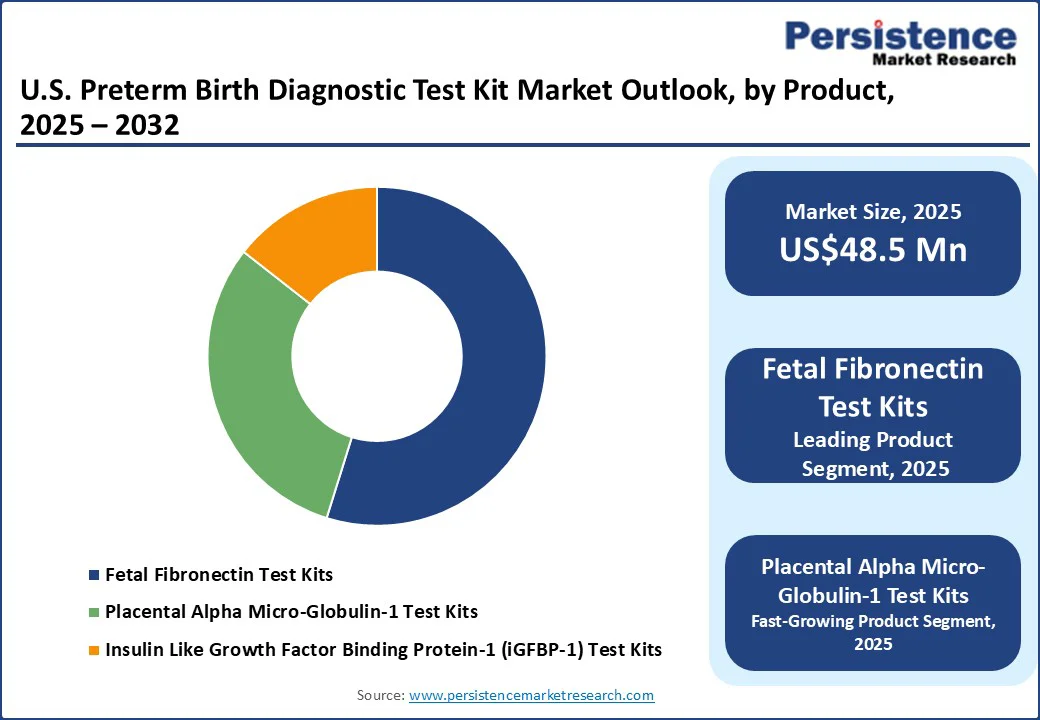
The U.S. preterm birth diagnostic test kit market size is likely to be valued at US$48.5 Mn in 2025 and is expected to reach US$74.4 Mn by 2032, growing at a CAGR of 6.3% during the forecast period from 2025 to 2032, driven by the rising awareness of the long-term risks associated with premature delivery.
Preterm birth, defined as the delivery before 37 weeks of gestation, affects nearly 400,000 infants annually in the U.S., placing a significant medical and emotional burden on families.
Key Industry Highlights:
| Key Insights | Details |
|---|---|
| U.S. Preterm Birth Diagnostic Test Kit Market Size (2025E) | US$48.5 Mn |
| Market Value Forecast (2032F) | US$74.4 Mn |
| Projected Growth (CAGR 2025 to 2032) | 6.3% |
| Historical Market Growth (CAGR 2019 to 2024) | 5.9% |
As per the World Health Organization, preterm birth remains a pressing public health challenge in the U.S. In 2022, preterm birth affected nearly 1 in every 10 infants born, contributing to nearly 14% of all infant deaths before 1 year of age (CDC, 2024).
Babies born too early, particularly before 32 weeks, face severe risks, including developmental delays, cerebral palsy, respiratory distress, and long-term disability. Beyond the human toll, preterm births impose a staggering economic burden of over US$26 Bn annually (US$65,000 per preterm birth) on the U.S. healthcare system.
The rising incidence and associated costs underscore the urgent need for accurate, early diagnostic tools such as fetal fibronectin (fFN), Placental Alpha Micro-Globulin-1 (PAMG-1), and Insulin Like Growth Factor Binding Protein-1 (iGFBP-1) kits.
These test kits allow clinicians to identify pregnancies at risk to improve triage decisions and guide timely interventions. Consequently, the high prevalence of preterm birth and its health and financial impact strongly drive the U.S. preterm birth diagnostic test kit market.
Despite advancements in diagnostic testing, premature delivery remains the primary cause of death for children under five worldwide. The WHO classifies preterm birth into extremely preterm (<28 weeks), very preterm (28 to <32 weeks), and moderate to late preterm (32 to 37 weeks), depending on the gestational age. Several diagnostic tests currently available in the market exhibit variability in accuracy and clinical interpretation, limiting their reliability.
Various studies demonstrate moderate sensitivity and specificity of existing biomarkers such as fFN and PAMG-1. This may be attributed to factors such as sample collection and processing techniques, among others, resulting in inconsistencies across clinical settings.
Moreover, the multifactorial nature of preterm birth complicates the interpretation of test results, as no single biomarker can encompass all potential risk factors, making definitive decisions a challenge. Addressing these inconsistencies is crucial for enhancing the clinical utility of preterm birth diagnostic tests.
The growing advancements in blood-based biomarkers present significant opportunities for the U.S. preterm birth diagnostic test kit market. The FDA-cleared PreTRM® test by Sera Prognostics analyzes proteins in maternal blood during weeks 18-20 of pregnancy to assess the risk of spontaneous preterm birth, enabling personalized clinical decisions.
Developments and integration of the cell-free nucleic acids (cfRNA), proteomics, and circulating microparticles are enhancing prenatal care in the U.S. A recent multi-site U.S. study identified a 7-marker set of CMP (circulating microparticle)-derived proteins from the first trimester of pregnancy to stratify pregnant patients according to their risk for spontaneous preterm birth (sPTB).
Similarly, another research identified novel cell-free RNAs (cfRNAs) in maternal plasma in combination with placental RNA profiles. These cfRNAs act as preterm biomarkers to validate and predict preterm birth, offering noninvasive and earlier diagnostic potential.
Growing emphasis on preventive prenatal health and integration of blood-based tests into routine screening is likely to improve early intervention, reduce preterm birth rates, and cut costs associated with late-stage neonatal care.
Fetal Fibronectin (fFN) test kits dominate the U.S. preterm birth diagnostic test kit market. These kits are widely used in hospitals and outpatient maternity clinics to assess symptoms of preterm labor. Their strong evidence base, long-standing clinical acceptance, and FDA clearance for predicting preterm delivery risk have made fFN tests an integral part of obstetric guidelines.
Additionally, established reimbursement frameworks and a legacy of clinical data demonstrating high negative predictive value have helped fFN test kits retain the largest market share compared to newer biomarker-based alternatives.
Vaginal discharge samples remain the dominant segment. The leading commercial products currently available in the U.S. preterm birth diagnostic test kit market, such as fFN, PAMG-1, and IGFBP-1, are validated for cervicovaginal fluid testing.
Clinically, cervicovaginal secretions provide the most reliable biomarkers associated with preterm labor and membrane rupture, ensuring higher diagnostic accuracy than urine or blood samples. The collection process is also simple and minimally invasive, suitable for triage as well as routine evaluations of preterm birth, thereby remaining the standard and dominant sample source across U.S. clinical practice.
The rising demand for rapid decision-making and effective triage in emergency and maternity units has significantly increased the adoption of Point-of-Care (POC) testing, establishing it as an emerging technology segment in the market.
POC test kits are easy to use, provide actionable results within minutes, and improve workflow efficiency without requiring complex laboratory infrastructure. This enables timely interventions and helps reduce unnecessary hospitalizations. Additionally, the growing availability of FDA-approved POC kits, coupled with payer interest in cost-effective care, is driving accelerated adoption, positioning POC testing as a key driver of market growth.
There is a growing integration of prenatal diagnostics into value-based care (VBC) models in the U.S. The VBC model encompasses programs that emphasize accountable care, improved outcomes, cost-efficiency, and provide financial incentives for patient improvement. This aligns well with preterm birth prevention since complications cost the healthcare system over US$26 Bn annually.
Diagnostic kits such as fFN and PAMG-1 are increasingly positioned as tools that reduce unnecessary hospital admissions and enable earlier interventions. Payers, including CMS and private insurers, are exploring reimbursement models that reward preventive prenatal care, bundling diagnostics into maternity episodes of care. This payer-driven shift underscores diagnostics’ growing role in U.S. maternal health management under VBC frameworks.
The U.S. preterm birth diagnostic test kit market is a highly competitive sector, with established global diagnostics firms and specialized maternal health companies competing for market share. Leading players emphasize FDA approvals, strong clinical trial data, and strategic partnerships with hospitals to strengthen market presence. New entrants and innovators are exploring blood-based biomarkers and digital integration to differentiate offerings.
The U.S. preterm birth diagnostic test kit market size is projected to be valued at US$48.5 Mn in 2025.
The rising preterm birth rates and strong clinical adoption of FDA-cleared diagnostic kits drive the U.S. preterm birth diagnostic test kit market.
The U.S. preterm birth diagnostic test kit market is poised to witness a CAGR of 6.3% between 2025 and 2032.
Expansion of rapid point-of-care kits and integration of novel biomarkers into routine prenatal care present major opportunities.
Major players in the U.S. preterm birth diagnostic test kit market are Creative Diagnostics, QIAGEN Sciences LLC, Actim Oy, Hologic, Inc., and Sera.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Sample
By Technology
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author