ID: PMRREP25129| 206 Pages | 4 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

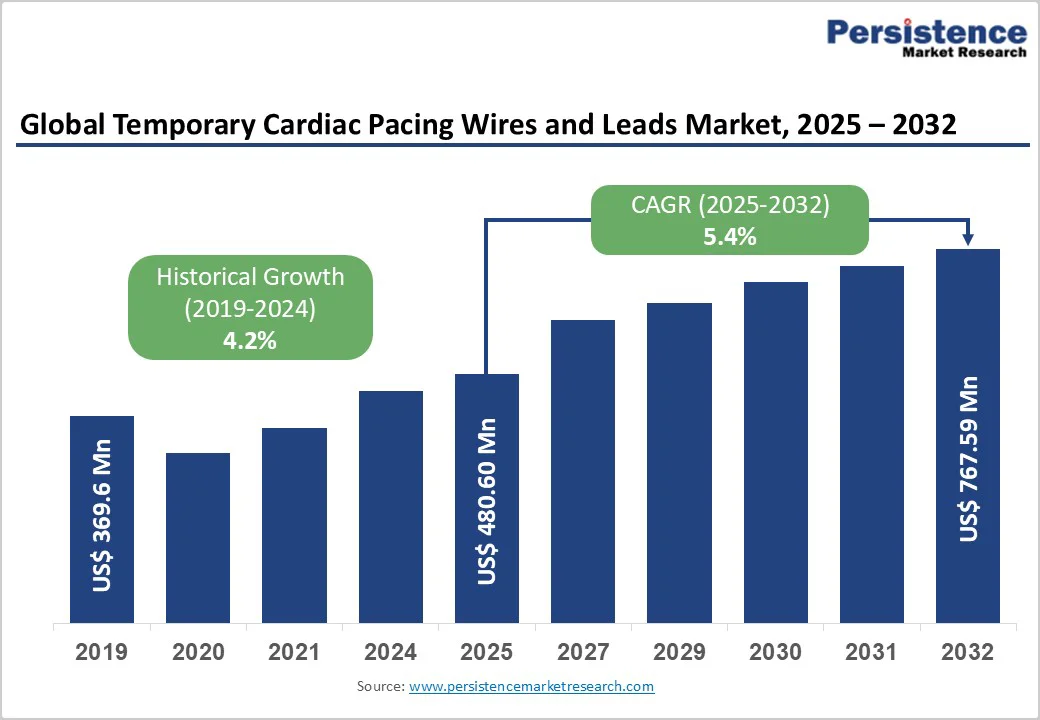
The global temporary cardiac pacing wires and leads market size is valued at US$480.6 million in 2025 and projected to reach US$767.6 million by 2032. The market is projected to record a CAGR of 5.4% from 2025 to 2032.
The demand for temporary cardiac pacing wires and leads is increasing as the prevalence of cardiovascular diseases, arrhythmias, and heart-failure–related complications rises worldwide. Growing geriatric populations, higher volumes of cardiothoracic surgeries, and expanding use of temporary pacing during perioperative stabilization are driving wider clinical adoption.
| Key Insights | Details |
|---|---|
|
Market Size (2025E) |
US$ 480.60 Mn |
|
Market Value Forecast (2032F) |
US$ 767.59 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.2% |
Rising cardiovascular disease (CVD) incidence is a key driver of the temporary cardiac pacing wires and leads market. As global CVD cases continue to increase, a growing number of patients require temporary pacing support during cardiothoracic surgeries, perioperative stabilization, heart-failure management, and arrhythmia correction. The expanding disease burden driven by ageing populations and increasing metabolic risks such as obesity and diabetes continues to strengthen demand for temporary pacing solutions across both developed and emerging healthcare systems.
For instance, in 2025, the British Heart Foundation reported that approximately 640 million people worldwide are living with CVD, equivalent to nearly 1 in 12 individuals globally. This number has been steadily increasing due to shifting lifestyles, a growing and ageing population, and improved survival rates following heart attacks and strokes, indicating that the burden will continue to rise in the coming years.
Technological advancements in lead design and disposable pacing systems are significantly driving market growth. Manufacturers are developing next-generation temporary pacing leads with improved biocompatibility, flexible polymer materials, enhanced insulation, and advanced tip-fixation mechanisms to reduce the risk of dislodgement, infection, and pacing failure. For instance, in July 2024, researchers from the Massachusetts Institute of Technology’s Department of Mechanical Engineering introduced a 3D-printable bioadhesive pacing lead capable of directly interfacing with cardiac tissue. This innovation enables minimally invasive adhesive implantation and incorporates a gentle detachment mechanism, offering a safer and more tissue-friendly alternative to traditional temporary pacing leads.
Infection, lead dislodgement, and pacing-related complications remain major restraints in the temporary cardiac pacing wires and leads market. Temporary pacing systems carry risks such as bloodstream infections, lead displacement, bleeding, cardiac perforation, and arrhythmia induction, which increase hospital stays, raise clinical workload, and prompt cautious adoption. These safety concerns often lead clinicians to prefer alternative stabilization methods when possible, limiting broader utilization in some settings.
High device prices and elevated procedural costs further constrain market growth. Temporary pacing leads require specialized materials, sterile handling, and trained personnel, making overall treatment expensive for hospitals, especially in cost-sensitive regions. Limited reimbursement for temporary pacing procedures in several countries adds additional financial pressure, reducing procurement frequency and slowing the adoption of advanced, higher-priced pacing technologies.
The growing shift toward single-use and anti-infection disposable pacing leads to significant opportunities in the market. Hospitals are increasingly adopting sterile, single-use temporary pacing wires to minimize cross-contamination, prevent catheter-related bloodstream infections, and streamline infection-control protocols. Stricter hospital safety standards and infection-prevention guidelines continue to accelerate demand for disposable, antimicrobial-coated, and biocompatible pacing solutions.
For example, in April 2025, engineers at Northwestern University developed an injectable, rice-sized biodegradable pacemaker designed for temporary pacing needs. The device pairs with a soft, flexible, wireless wearable controller that detects irregular heart rhythms and activates the pacemaker using light pulses that penetrate the skin and surrounding tissues. Tailored for patients, including newborns with congenital heart defects who require only short-term pacing, the fully dissolvable pacemaker eliminates the need for surgical extraction.
Innovations in minimally invasive pacing technologies and improved fixation mechanisms also offer significant growth opportunities. New lead designs featuring enhanced tip fixation, flexible polymer structures, and atraumatic materials aim to reduce dislodgement, improve procedural accuracy, and support safer perioperative pacing. The development of low-profile, adhesive, and image-guided temporary pacing systems enables easier placement and removal, positioning these next-generation devices as key opportunities for manufacturers targeting surgical, emergency, and critical-care settings.
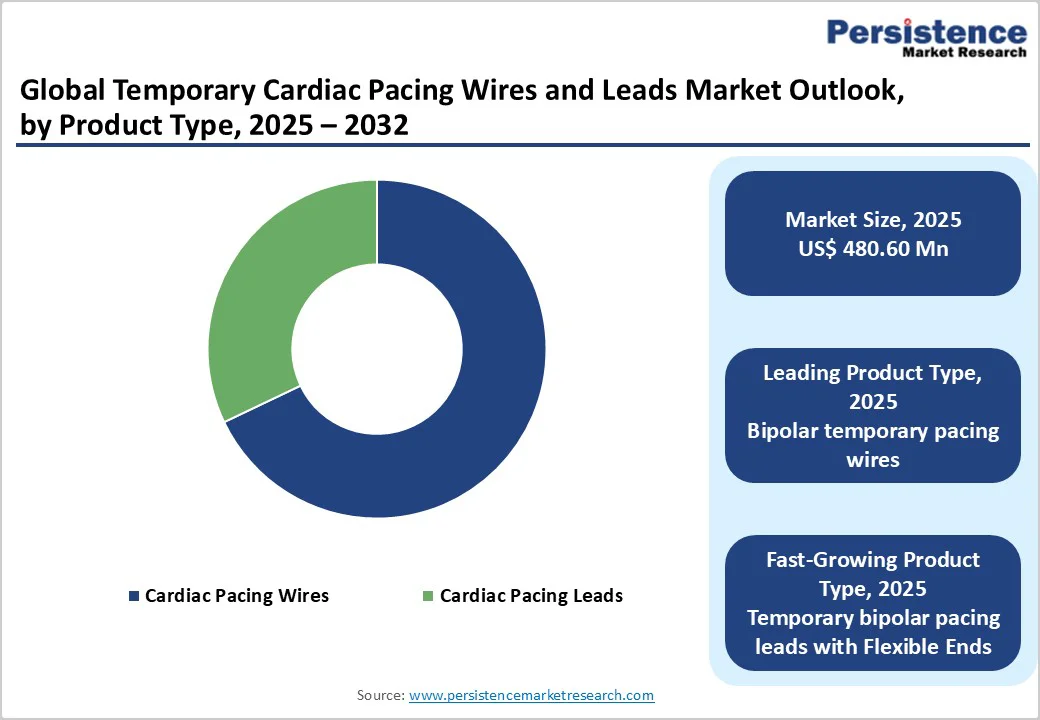
The cardiac pacing leads stimulators segment is projected to dominate the global temporary cardiac pacing wires and leads market in 2025, accounting for 27.7% of revenue. The segment’s strong performance is primarily due to its higher clinical use across emergency, perioperative, and ICU settings, offering more reliable sensing, improved stability, and greater compatibility with external pacemakers than pacing wires. Their adoption is strengthened by the growing volume of cardiothoracic surgeries and acute conduction-disturbance cases that require dependable temporary pacing support. Additionally, bipolar and specialty leads (flexible-end or balloon-tipped) are increasingly preferred because they reduce lead displacement, improve maneuverability, and enable safer short-term cardiac rhythm management.
The cardiothoracic surgeries segment is projected to dominate the global temporary cardiac pacing wires and leads market in 2025, accounting for 59.5% of revenue. This is due to their routine requirement for temporary pacing support during and after open-heart procedures, making pacing wires and leads a standard part of post-operative rhythm management. These surgeries frequently involve risks of bradyarrhythmias, AV blocks, and conduction disturbances, driving consistent use of temporary epicardial or transvenous pacing systems. The high global volume of coronary artery bypass grafting (CABG), valve repair, replacement, and congenital heart surgeries.
The hospitals segment is projected to dominate the global temporary cardiac pacing wires and leads market in 2025, accounting for 64.2% of revenue. This is due to their high concentration of cardiothoracic surgeries, advanced critical-care units, and emergency departments that routinely require temporary cardiac pacing for acute rhythm stabilization. Hospitals manage the largest patient volumes for cardiac surgery, myocardial infarction, conduction disorders, and ICU monitoring, making them the primary purchasers of temporary pacing wires and leads. Their better-equipped cath labs, surgical theaters, and 24/7 cardiac care capabilities further drive this segment’s growth.
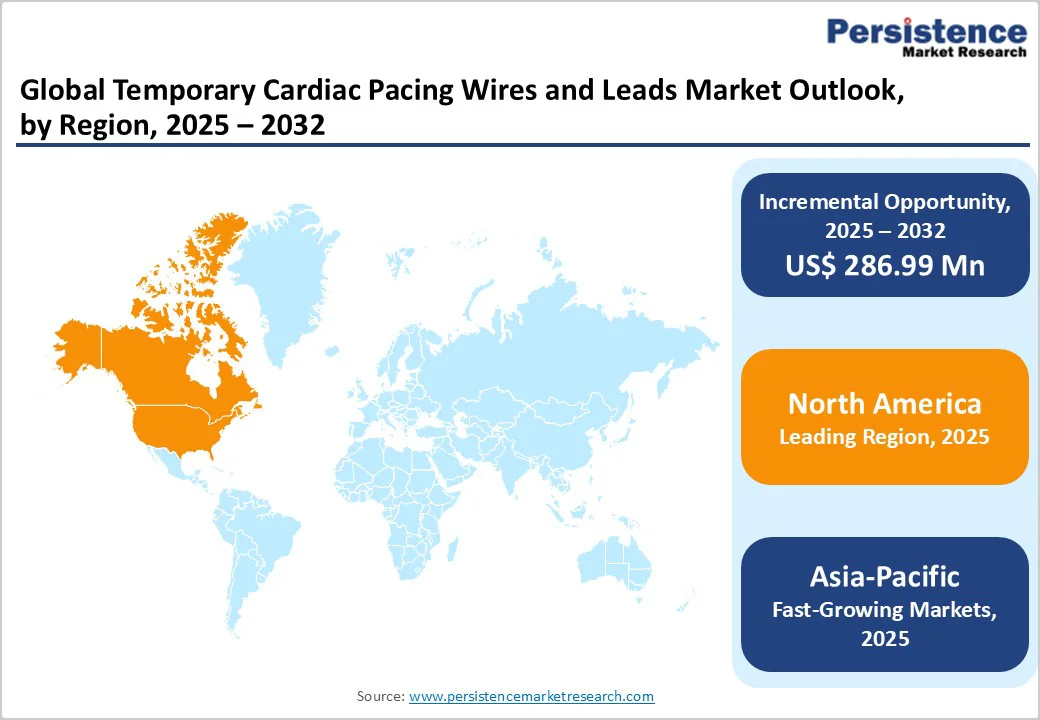
The North America market is expected to dominate globally with a value share of 43.4% in 2025, with the U.S. leading the region due to a combination of advanced healthcare infrastructure, a high volume of cardiothoracic surgeries, and well-established cardiac care units. Hospitals and specialty cardiac centers in the U.S. are increasingly adopting technologically advanced temporary pacing wires and leads, including bipolar, flexible-end, and balloon-tipped options, which improve safety, maneuverability, and procedural success.
Additionally, strict clinical guidelines for cardiac rhythm management, proactive postoperative monitoring, and rising awareness of complications such as bradyarrhythmias or conduction blocks drive consistent use. Favorable reimbursement policies, comprehensive insurance coverage, and high patient access to cardiac care further reinforce North America’s dominance. The presence of key device manufacturers, ongoing R&D, and continuous adoption of disposable, infection-reducing temporary pacing products
The European market is expected to grow steadily, due to well-established healthcare systems, high standards of cardiac care, and widespread adoption of advanced pacing technologies. Countries such as Germany, France, and the U.K. lead in cardiothoracic surgery volumes and post-operative rhythm management, driving demand for temporary pacing wires and leads. Strong regulatory frameworks and hospital compliance programs encourage the use of safer, single-use, and antimicrobial-coated devices.
Moreover, government initiatives to improve patient outcomes and reduce post-surgical complications, along with growing awareness of arrhythmia management, are further supporting market growth in the region. Additionally, the presence of key global and regional manufacturers offering innovative products and partnerships with academic institutions for training and research are enhancing the adoption of temporary pacing solutions across major European hospitals. Rising private healthcare investments and the trend toward minimally invasive cardiac procedures are boosting market growth in the region.
The Asia Pacific market is expected to register a relatively higher CAGR of around 7.3% between 2025 and 2032, driven by the rising incidence of cardiovascular diseases, increasing cardiothoracic surgeries, and expanding healthcare infrastructure. Countries such as China, India, and Japan are witnessing a surge in demand for temporary pacing solutions due to growing populations, aging demographics, and increased access to tertiary care centers. Additionally, government healthcare initiatives, rising awareness of arrhythmia and post-surgical care, and investments by global device manufacturers in regional distribution are accelerating adoption.
The adoption of technologically advanced pacing leads, such as bipolar and balloon-tipped leads, is increasing across hospitals and specialty clinics. Furthermore, collaborations between international device companies and local healthcare providers are improving product accessibility and affordability.
The global temporary cardiac pacing wires and leads market is highly competitive, with major players such as Medtronic, Merit Medical Systems Inc., BD, B. Braun SE, Abbott, and Edwards Lifesciences Corporation dominating through broad cardiac care portfolios, strong distribution networks, and long-standing expertise in pacing technologies. These companies offer a wide range of temporary pacing wires, transvenous leads, introducers, and perioperative pacing solutions widely used in cardiothoracic surgeries and emergency cardiac care.
Manufacturers are increasingly focusing on safer, more flexible, and infection-resistant pacing solutions, including single-use epicardial wires, balloon-tipped transvenous leads, biocompatible coatings, enhanced fixation systems, and low-profile temporary pacing platforms. Strategic initiatives such as mergers & acquisitions, expansion of cardiac device manufacturing capabilities, clinical research partnerships, and the development of dissolvable or minimally invasive pacing technologies are further strengthening competitive positioning across global markets.
Key Industry Developments:
The global temporary cardiac pacing wires and leads market is projected to be valued at US$ 480.60 Mn in 2025.
Rising cardiovascular disease burden, increasing cardiothoracic surgical volumes, and growing need for perioperative and emergency pacing are driving the global temporary cardiac pacing wires and leads market.
The global temporary cardiac pacing wires and leads market is poised to witness a CAGR of 5.4% between 2025 and 2032.
Development of single-use, anti-infection pacing leads and innovations such as bioadhesive, dissolvable, and minimally invasive temporary pacing systems are creating strong growth opportunities in the market.
Medtronic, Merit Medical Systems Inc., BD, B. Braun SE, Abbott, and Edwards Lifesciences Corporation are some of the key players in the temporary cardiac pacing wires and leads market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn Volume in Units (If Applicable) |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Application
By Technique
By Age Group
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author