ID: PMRREP35936| 196 Pages | 8 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

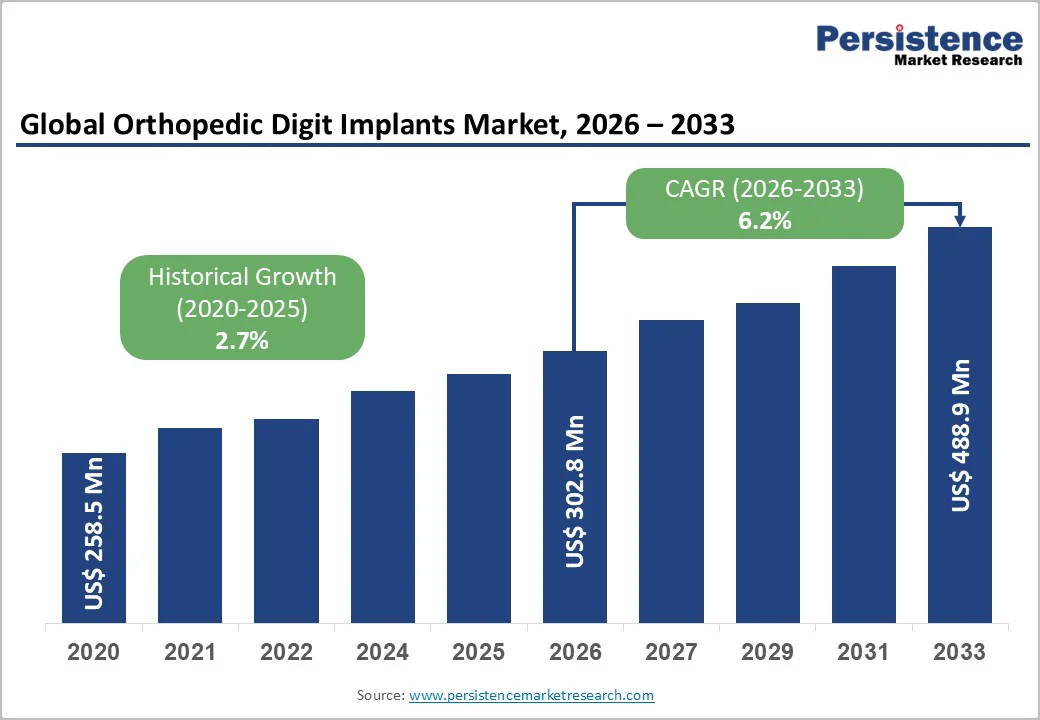
The global orthopedic digit implants market size is likely to be valued at US$302.8 million in 2026. It is expected to reach US$488.9 million by 2033, growing at a CAGR of 6.2% during the forecast period from 2026 to 2033, driven by the rising burden of osteoarthritis, an expanding elderly population, and a growing number of hand and foot trauma cases.
Increased adoption of minimally invasive digit-preserving procedures, along with advancements in materials engineering and 3D-printed implants, strengthens the mid-single-digit growth outlook. The strongest commercial momentum is observed in North America and advanced Asia-Pacific healthcare ecosystems, while broader adoption is expected as reimbursement improves and patient-specific implants gain regulatory traction.
| Key Insights | Details |
|---|---|
| Orthopedic Digit Implants Market Size (2026E) | US$302.8 Mn |
| Market Value Forecast (2033F) | US$488.9 Mn |
| Projected Growth (CAGR 2026 to 2033) | 6.2% |
| Historical Market Growth (CAGR 2020 to 2025) | 2.7% |
The rapid growth of the global elderly population continues to drive a significant rise in osteoarthritis cases, particularly in small joints of the hand and foot. Epidemiological studies indicate that hundreds of millions of people worldwide are affected by osteoarthritis, with prevalence increasing sharply between 2010 and 2020.
As conservative treatment options lose effectiveness in advanced disease stages, more patients require surgical reconstruction using digit implants. The rising incidence of degeneration in metatarsophalangeal (MTP), metacarpophalangeal (MCP), and proximal interphalangeal (PIP) joints increases the volume of implant procedures.
Technological advancements, including additive manufacturing, enhanced titanium alloys, pyrocarbon, and modern minimally invasive tools, are transforming digital implant performance and longevity.
Patient-specific 3D-printed implants for metatarsal and phalangeal joints entered early regulatory review during 2024 - 2025, indicating strong momentum toward broader commercial availability. Lattice structures that improve osseointegration, cartilage-mimicking surfaces, and precision instruments that reduce recovery time collectively enhance clinical outcomes and surgeon confidence.
Digit implants fall under the category of permanent implantable medical devices, which subjects them to some of the most rigorous regulatory standards in global healthcare systems.
Manufacturers must demonstrate long-term safety, biocompatibility, mechanical durability, and clinical performance through comprehensive pre-market trials and verification protocols. Regulators such as the FDA, EMA, and PMDA frequently require multi-year outcome data, fatigue testing, wear simulations, and detailed risk-benefit analyses.
Post-market surveillance obligations also remain extensive and costly, stretching from Unique Device Identification (UDI) tracking to real-world performance reporting.
Emerging materials such as pyrocarbon, porous titanium, and additively manufactured lattice structures often attract heightened scrutiny, leading to longer review cycles and higher R&D expenditures. These extended pathways slow product launches and increase barriers to entry for smaller companies.
Reimbursement policies for digit reconstruction vary widely across health systems and insurers. While conservative treatments are typically well-reimbursed, implant-based reconstruction often encounters inconsistent coverage, limiting adoption in certain markets. Regions with restrictive outpatient reimbursement exhibit lower procedure penetration than countries that support comprehensive coverage.
Patient-specific 3D-printed implants are positioned as one of the most transformative opportunities within the orthopedic digit implants market. These devices can be engineered to match a patient’s exact anatomical geometry, improving joint biomechanics, stability, and long-term functional outcomes.
Examples such as custom MTP joint replacements or individualized phalangeal resurfacing components demonstrate significant clinical potential for complex revision cases or patients with atypical anatomy.
Adoption momentum will rely on stronger clinical datasets, surgeon training in digital planning workflows, and scalable additive manufacturing hubs capable of meeting regulatory documentation standards. Payers are gradually recognizing the value of personalized implants as evidence emerges around reduced revision rates and faster recovery.
This trend supports a broader market premiumization cycle where hospitals and ambulatory centers selectively invest in customized, high-margin implant solutions.
Digit implant procedures are rapidly shifting from inpatient hospital environments to outpatient facilities and ambulatory surgery centers, reshaping case volumes and cost structures across the orthopedic ecosystem. ASCs enable same-day surgeries with shorter recovery times and lower perioperative costs, aligning with payer incentives and patient preferences for minimally invasive care.
Countries such as the U.S. and several European markets are witnessing strong ASC expansion, particularly in hand and foot surgery networks.
A transition of even 10 percent of inpatient procedures to outpatient settings can generate substantial savings for healthcare systems while freeing hospital capacity for higher-acuity cases. This migration also increases annual procedure throughput, driving higher implant demand and accelerating adoption of streamlined surgical kits, sterilization-efficient instrumentation, and compact implant systems designed specifically for ambulatory workflows.
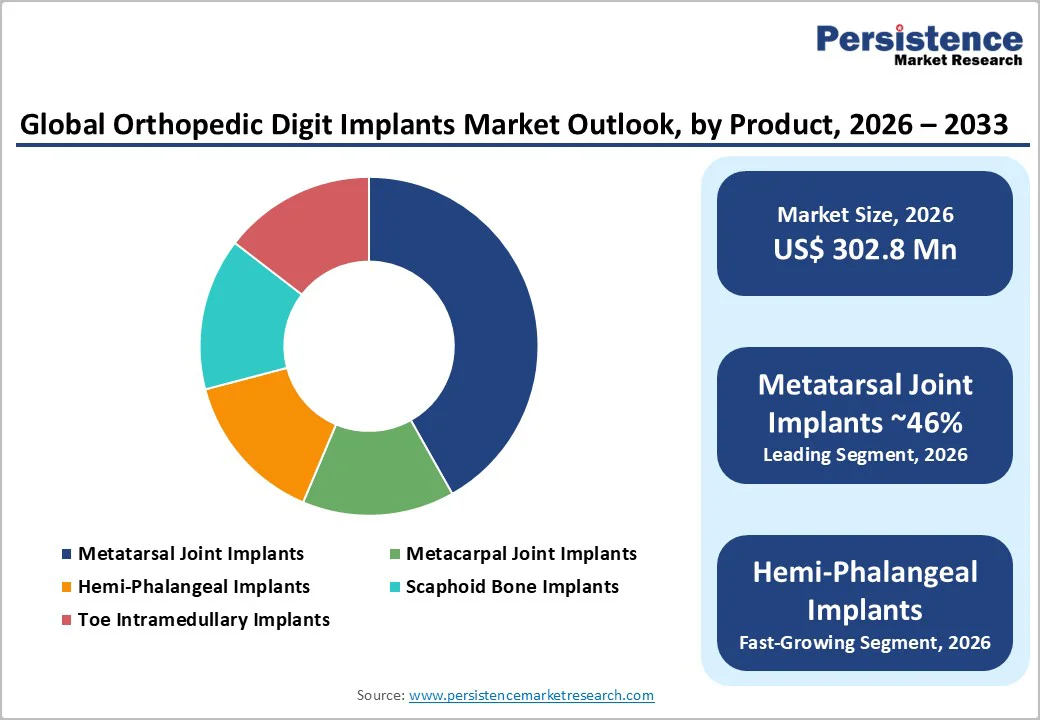
Metatarsal joint implants are anticipated to account for the largest share, roughly 46%, of the market in 2026. Their dominance reflects the substantial incidence of forefoot deformities, including hallux rigidus, post-traumatic degeneration, and metatarsal arthritis that often requires surgical reconstruction. Many procedures involve multiple components or bilateral interventions, which increases per-patient implant utilization.
Surgeons are also more familiar with MTP arthroplasty compared with smaller finger joints, reinforcing preference for standardized metatarsal systems. Innovation is expanding segment strength; several manufacturers have introduced 3D-printed MTP implants tailored to patient anatomy, improving fixation and long-term kinematics.
For example, custom titanium MTP replacements used in complex hallux rigidus cases have shown promising early clinical outcomes and shortening of recovery timelines. These advances, together with high procedural frequency in both sports medicine and general orthopedics, secure the segment’s continued leadership within product categories.
Hemi-phalangeal implants are set to record the highest growth rate over the forecast period, driven by rising interventions for isolated PIP and DIP joint degeneration, finger fractures, and post-traumatic deformities. Demand is increasing among younger and active patients who prefer motion-preserving procedures over full arthrodesis.
Modern implant designs incorporate improved curvature matching, refined stem fixation, and wear-resistant biomaterials that provide functional mobility with minimal revision risk.
Adoption is particularly strong in specialized hand-surgery centers, where outpatient procedures for sports injuries or repetitive-strain conditions are common. For instance, hand units treating climbing-related and workplace digital injuries have reported growing preference for hemi-phalangeal implants that restore functional grip without compromising joint stability.
Titanium is expected to remain the leading material for small-joint implants in the upper and lower extremities, with a market share of 49% in 2026, owing to its exceptional biocompatibility, corrosion resistance, and favorable strength-to-weight profile.
It reliably supports osseointegration and maintains structural stability, even in high-load joints such as the first MTP. Most premium, modular, and patient-specific systems rely on titanium, particularly because the material is well-suited to additive manufacturing processes used to create porous, bone-mimicking geometries.
Titanium implants also benefit from predictable regulatory performance, with decades of clinical evidence underpinning safety and efficacy. A number of orthopedic companies now offer titanium patient-matched implants for complex foot reconstruction, enabling surgeons to restore alignment more accurately in revision or deformity cases.
Pyrocarbon and advanced composite materials are anticipated to expand at the fastest rate as clinical studies validate their cartilage-like wear behavior and natural articulation characteristics. Their elastic modulus more closely resembles cortical bone, which helps limit stress shielding and preserve surrounding tissue integrity. Pyrocarbon’s long history in finger joint replacements is gaining renewed attention as newer designs demonstrate smoother gliding surfaces and reduced implant abrasion.
Composite materials, such as reinforced polymer-carbon hybrids, are increasingly used in trial centers for motion-preservation implants aimed at younger patients.
Several hospitals have reported favorable functional scores in PIP and MTP reconstructions using pyrocarbon components, particularly in patients seeking pain reduction without losing range of motion. As outcome data accumulate and surgeon familiarity increases, adoption of pyrocarbon and composite-based implants is expected to accelerate in both orthopedic and hand-specialty facilities.
North America is expected to capture the largest market share at roughly 49.5%, driven primarily by the U.S., which maintains a dominant role in global procedure volumes and implant adoption.
This leadership stems from the high prevalence of osteoarthritis and trauma-related digit injuries, as well as the region’s strong orthopedic infrastructure, which includes specialized hand and foot surgery centers capable of handling substantial patient throughput.
Advanced implant technologies, including custom 3D-printed and motion-preserving devices, are widely utilized due to high per-capita healthcare spending and robust insurance coverage for indicated surgeries.
Surgeons across the U.S. benefit from well-structured fellowships and specialized training programs, fostering rapid uptake of innovative implant systems and minimally invasive techniques. Market strength is further reinforced by significant private-sector R&D investment and ongoing collaboration between device manufacturers and healthcare institutions, supporting steady product advancement.
A favorable regulatory environment also contributes to market stability and growth. Although the FDA requires rigorous evidence for novel materials and 3D-printed implants, its predictable review pathways provide clarity for manufacturers.
Once devices obtain clearance, they typically secure strong reimbursement, accelerating clinical adoption. Additionally, the shift toward outpatient and ambulatory surgical settings increases regional capacity while reducing overall care costs.
Europe represents a mature, technologically advanced orthopedic digit implant market, sustaining steady mid-single-digit growth driven by demographic and clinical trends.
Aging populations in Germany, France, Italy, and the U.K. significantly increase demand for reconstruction procedures, while strong research ecosystems within major university hospitals promote the evaluation of new biomaterials, long-term clinical studies, and surgeon training. These capabilities collectively accelerate the adoption of next-generation small-joint implant solutions.
Performance varies across key countries. Germany leads the region with high surgical capacity, substantial procedure volumes, and rapid uptake of innovative digit reconstruction systems.
The U.K. operates under centralized NHS purchasing frameworks, where cost-effectiveness requirements shape adoption timelines. France and Spain continue to expand private-sector surgical networks and gradually shift toward outpatient care, enabling higher case throughput and broader utilization of small-joint implants.
Across Europe, demand is reinforced by robust clinical R&D activity, demographic pressures, and a measured transition into ambulatory surgical settings in select markets. Regulatory dynamics also influence market evolution.
The EU Medical Device Regulation (MDR) has raised evidence standards and lengthened certification processes, initially creating challenges for manufacturers. However, MDR improves safety, harmonizes compliance across member states, and heightens barriers to low-quality products, ultimately supporting the market for high-performance implant systems.
The Asia Pacific region is likely to be the fastest-growing market for orthopedic digit implants, fueled by rising healthcare expenditure, expanding access to surgical care, and rapid modernization of orthopedic infrastructure.
Market growth is reinforced by increasing patient awareness, the proliferation of specialized hand and foot surgery units, and the integration of advanced imaging and surgical navigation systems within major hospitals. Together, these shifts support a substantial expansion in digit reconstruction procedures across both urban and secondary healthcare markets.
Performance varies among major countries. China benefits from a large patient base, rapid growth in orthopedic capacity, and extensive domestic manufacturing that supports competitive pricing and broad availability.
Japan, by contrast, combines advanced reimbursement systems, a highly aging population, and a sophisticated clinical environment that naturally drives stable long-term demand for small-joint implants. India and ASEAN countries are experiencing rising procedure volumes driven by urbanization, growth in private hospitals, and greater affordability of orthopedic surgeries as insurance penetration improves.
Key growth drivers include rapid demographic aging, particularly in Japan and China, as well as significant investments in surgical training and the expansion of modern orthopedic facilities. Local production capabilities continue to strengthen, enabling cost-efficient implant solutions and collaborations with global manufacturers.
Regulatory environments vary widely across the region. Japan maintains a structured and mature review framework, while Southeast Asian markets continue to modernize approval pathways.

The global orthopedic digit implants market is moderately concentrated. Large orthopedic corporations hold substantial shares in high-value implant categories, supported by extensive distribution networks and strong clinical evidence. At the same time, numerous specialist companies focus on niche extremity segments, offering innovative designs and targeted instrumentation. Market concentration is driven by brand reputation, surgeon familiarity, and investment in product development and training.
Market leaders emphasize innovation in materials and 3D printing, targeted acquisitions to expand product lines, and distribution partnerships to enhance global reach. Surgeon education, comprehensive clinical evidence, and bundled surgical solutions (implant + instruments + planning tools) remain central competitive differentiators.
The global orthopedic digit implants market size is estimated to reach US$302.8 million in 2026.
By 2033, the market value is projected to reach US$488.9 million.
Key trends include growing adoption of 3D-printed, patient-specific implants for both hand and foot reconstruction and a rise in motion-preserving procedures, especially in younger and active patient groups.
In material type, titanium remains the dominant material with over 50% share, supported by its biocompatibility, mechanical strength, and compatibility with additive manufacturing.
The orthopedic digit implants market is expected to grow at a CAGR of 6.2% from 2026 to 2033.
Major players include Stryker Corporation, Zimmer Biomet, Integra LifeSciences, Arthrex, Inc., and Acumed LLC.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Material Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author