ID: PMRREP11542| 199 Pages | 2 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

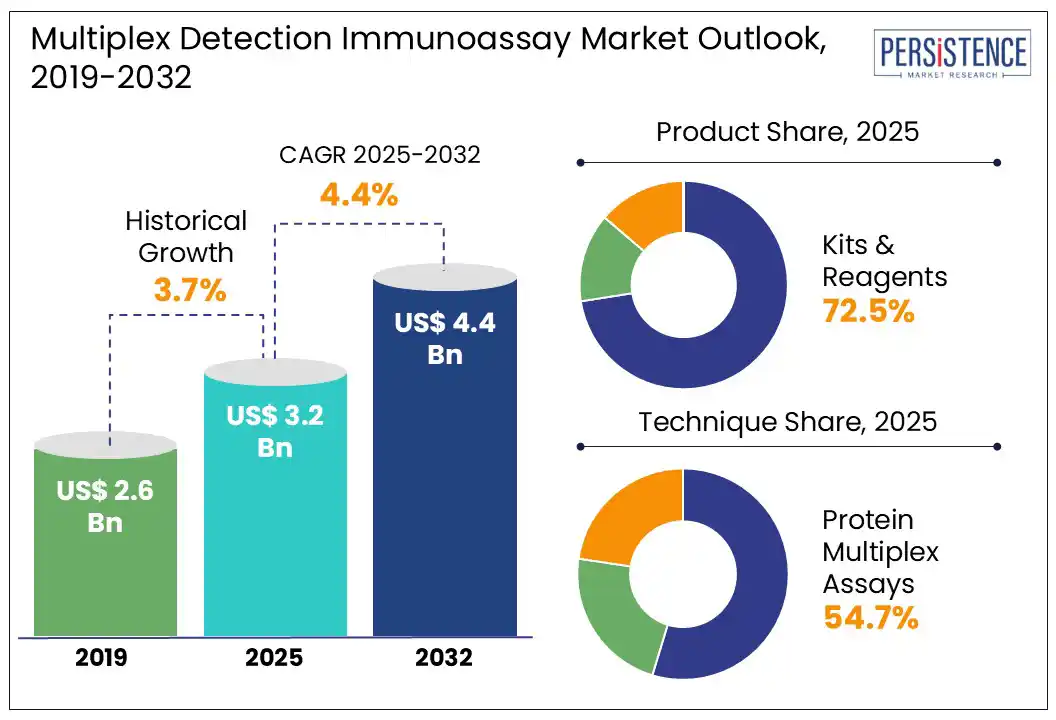
According to the Persistence Market Research report, the global multiplex detection immunoassay market size is likely to be valued at US$ 3.2 Bn in 2025 and is expected to reach US$ 4.4 Bn by 2032, growing at a CAGR of 4.4% during the forecast period from 2025 to 2032.
Multiplex detection immunoassays are revolutionizing diagnostics by enabling the simultaneous identification of multiple biomarkers in a single test, enhancing efficiency, reducing costs, and conserving sample volume. These technologies are especially valuable in complex diseases such as cancer, where molecular heterogeneity influences treatment decisions.
Advances in digital pathology, AI-driven image analysis, and multiplexed immunostaining have improved diagnostic accuracy. Innovations in ELISA and fluorescence immunoassays are streamlining workflows and supporting more precise, early, and personalized healthcare. Additionally, integration into electronic health records (EHR), is facilitating seamless data sharing, improving diagnostic precision and patient outcome.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Multiplex Detection Immunoassay Market Size (2025E) |
US$ 3.2 Bn |
|
Market Value Forecast (2032F) |
US$ 4.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
4.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.7% |
The growing need for rapid, accurate, and comprehensive diagnostic testing is fuelling the adoption of multiplex immunoassays. Unlike traditional single-analyte assays, multiplex platforms simultaneously detect multiple biomarkers in a single reaction. Bead-based multiplex immunoassays use uniquely color-coded beads conjugated with specific antibodies, enabling precise and parallel detection of numerous targets.This efficiency makes it ideal for both clinical diagnostics and research applications.
Additionally, rising demand for rapid point-of-care diagnostics in non-laboratory settings is driving the multiplex detection immunoassay market growth, as these tests offer enhanced patient convenience and faster results. Ongoing improvements in assay quality and performance further support their adoption. Moreover, multiplex immunoassays streamline drug development by allowing simultaneous analysis of multiple biomarkers, cutting down processing time, lowering costs, and sample requirements. This ability to efficiently monitor complex diseases is accelerating their integration in pharmaceutical research, clinical trials, and broader healthcare applications.
A key restraint for the multiplex detection immunoassay market is the inherent complexity and challenges in achieving accurate multiplexing. While immunoassays are effective for biomarker quantification, limitations in dynamic range, analytical sensitivity, and target specificity persist.
Developing high-performance multiplex assays requires identifying highly specific antibody pairs to avoid cross-reactivity, where antibodies bind non-target proteins, compromising assay accuracy. This is particularly difficult when analyzing multiple analytes with varying concentrations in the same assay, as maintaining linearity and selectivity across a wide range is critical.
Additionally, multiplex assays demand careful optimization of buffer conditions and reagents to minimize interference between targets, often extending development time compared to singleplex assays. Platform-specific antibody validation is essential, as antibodies that perform well in singleplex may not maintain quality in multiplex formats.
Variability in technologies, reagents, and detection methods further complicates assay standardization. While these technical complexities slow assay development timelines, ongoing innovations in antibody engineering, assay design, and validation strategies are helping to overcome these barriers, supporting the continued advancement and broader adoption of multiplex immunoassays.
The development of custom multiplex immunoassays presents a significant opportunity in therapeutic development by enabling simultaneous detection and quantification of multiple biomarkers within a single sample. These specialized assays improve efficiency by reducing sample volume and providing comprehensive data from one experiment, critical in complex disease monitoring and drug development.
Recent advancements, such as Nonagen Bioscience’s Oncuria® platform, showcase the clinical utility of multiplex immunoassays in bladder cancer, offering non-invasive, algorithm-driven diagnostic solutions that have secured CMS (Centers for Medicare & Medicaid Services) reimbursement in January 2024. Strategic collaborations, such as Nonagen’s partnership with Protara Therapeutics in March 2025, to develop in-vitro diagnostic tests for clinical trials, highlightz the growing role of custom multiplex assays in patient stratification and therapy optimization.
Furthermore, ongoing research in multiplexed nanobiosensors and integrated biosensor array for multiplex biomarkers cancer diagnosis is expanding assay sensitivity and specificity, reinforcing their potential in personalized medicine and accelerating therapeutic development. This trend is expected to drive increased adoption and innovation in the global multiplex detection immunoassay market.
Kits & Reagents represent the most widely used product category in the global multiplex detection immunoassay market. These consumables are required for every test, ensuring consistent and reliable assay performance. Compared to instruments or software and services, kits and reagents have a broader user base, including research labs and clinical settings, leading to higher repeat purchases. Additionally, they are more cost-effective and easier to adopt without significant infrastructure investment, driving their widespread use and sustained demand globally.
Nucleic acid multiplex assays are projected to top the global market, accounting for nearly 54.7% share in 2025 due to their ability to simultaneously detect multiple genetic targets with high sensitivity and specificity. These assays are essential in infectious disease diagnostics, genetic testing, and personalized medicine. Their rapid turnaround time and capability to analyze complex genetic information in a single test make them highly efficient and cost-effective. Growing demand for advanced molecular diagnostics and outbreak monitoring further drives their market dominance.
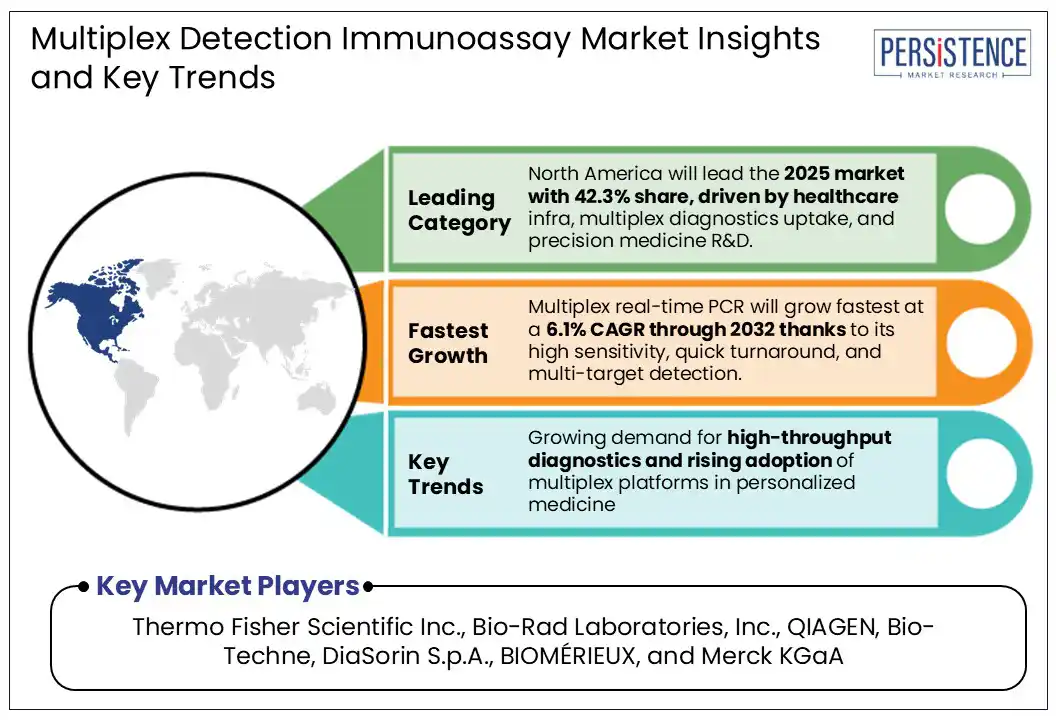
North America is projected to dominate the global market with over 40% revenue share in 2025. The region's robust healthcare infrastructure, high healthcare expenditure, and emphasis on adoption of advanced diagnostic technologies drive this growth. The United States plays a central role in this dominance, supported by extensive research initiatives, substantial government funding, and the presence of several leading providers of multiplex and proteomic assay services.
In September 2023, The National Institutes of Health established the Multi-Omics for Health and Disease Consortium awarding $11 million in its first year to advance multi-omic data generation and analysis. Additionally, in August 2024, Biognosys partnered with Alamar Biosciences to launch NULISA multiplex assay services, further strengthening the nation’s position in biopharma research.
The U.S. multiplex detection immunoassay market continues to expand rapidly due to growing demand for early disease detection, personalized medicine, and high-throughput and efficient diagnostic solutions, positioning it as a key contributor to the region’s market leadership.
Europe’s multiplex detection immunoassay market is rapidly advancing, fuelled by strategic collaborations and technological innovation. The joint venture between Seegene and Werfen in Spain in March 2024, exemplifies regional efforts to combine expertise and expand diagnostic capabilities.
Furthermore, cutting-edge platforms - EYRA system - introduced by the Swedish firm Mabtech in partnership with Qamcom’s in April 2025, offer automation-ready, no-contact, image-based multiplex solutions that enhance immunology research and antibody-based vaccine development.
Backed by a strong research infrastructure and increasing demand for efficient diagnostics, Europe is positioned as a key hub for multiplex immunoassay adoption, driven by its focus on collaborative innovation and advanced technology integration.
Asia Pacific is emerging as the lucrative market for multiplex detection immunoassay driven by increasing healthcare expenditure, expanding patient populations, and rising prevalence of chronic and infectious diseases.
Rapid advancements in healthcare infrastructure and growing awareness about early disease diagnosis are boosting the adoption of multiplex immunoassay technologies across the region. Countries such as China, India, and Japan are investing heavily in public and private research and development (R&D), facilitating the introduction of innovative diagnostic solutions. In 2024, South Korea allocated 4.96% of its GDP to R&D, whilst China and Japan committed 2.68% and 3.65% respectively.
Additionally, government initiatives promoting personalized medicine and improved healthcare access such as Japan’s Cancer Genomic Medicine (CGM) program and China’s Precision Medicine Initiatives are accelerating market growth. The increasing demand for cost-effective, high-throughput diagnostic tools further positions Asia Pacific as a key growth hub in the global market.
The global multiplex detection immunoassay market is a highly competitive marked by rapid innovation in multiplex PCR and digital assay technologies, with players focusing on enhanced sensitivity, automation, and high-order multiplexing to support advanced diagnostics and precision research.
The global market is set to reach US$ 3.2 Bn in 2025.
The market is projected to record a CAGR of 4.4% during the forecast period from 2025 to 2032.
Increasing need for simultaneous multi-biomarker detection and rapid, advancements in immunoassay technologies and expanding applications in drug development and disease monitoring fuels the global market.
Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., QIAGEN, Bio-Techne, DiaSorin S.p.A., BIOMÉRIEUX, and Merck KGaA are a few leading players.
North America is projected to dominate the global market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Technique
By Technology
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author