ID: PMRREP33136| 196 Pages | 2 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

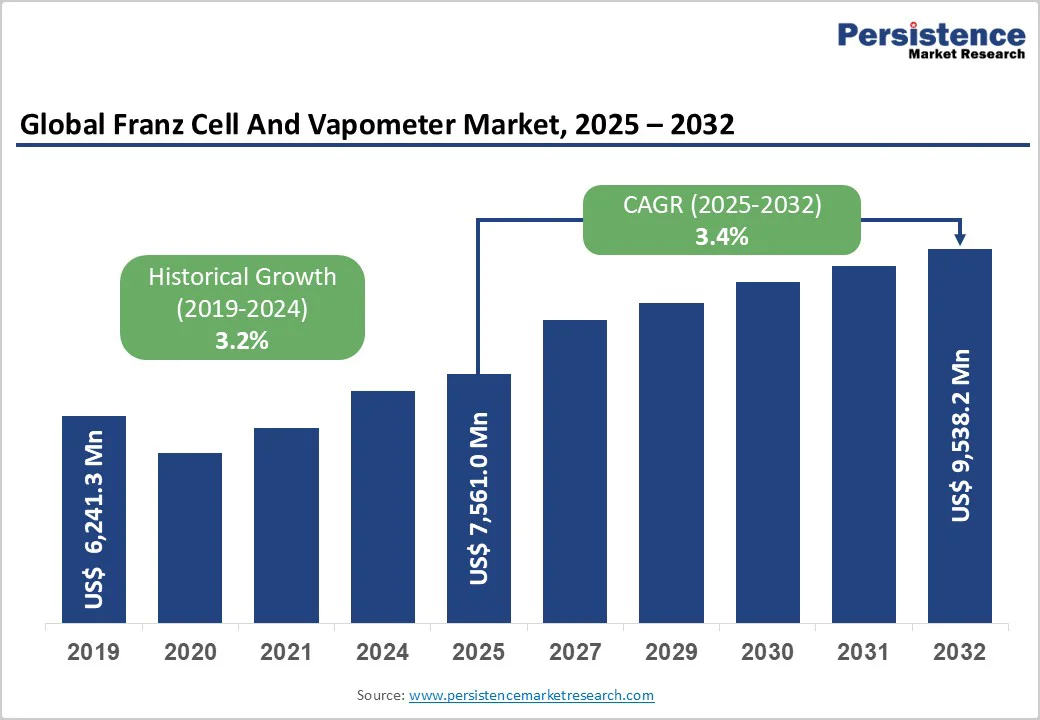
The global Franz cell and vapometer market size is valued at US$7,561.0 million in 2025 and is projected to reach US$9,538.2 million, growing at a CAGR of 3.4% during the forecast period from 2025 to 2032.
The Franz cell and vapometer market is expanding as pharmaceutical, cosmetics, and skincare companies intensify their focus on precise permeation and transepidermal water loss (TEWL) measurements. Growth is driven by rising demand for high-accuracy in-vitro diffusion testing to support topical drug development, biosimilar evaluation, and regulatory-compliant product validation. Advanced automated Franz cells, digital vapometers, and temperature-controlled diffusion systems are gaining traction for delivering reproducible data and faster R&D timelines.
| Key Insights | Details |
|---|---|
|
Franz Cell and Vapometer Market Size (2025E) |
US$7,561.0 Mn |
|
Market Value Forecast (2032F) |
US$9,538.2 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
3.4% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.2% |
Transdermal drug products have seen increased usage for various health conditions such as osteoporosis, contraception, chronic pain, nausea, and vomiting caused by chemotherapy, neurological disorders such as attention-deficit hyperactivity disorder, depressive disorders, and many others. Franz cells and vapometers are widely used to evaluate the efficacy of transdermal drugs. In the cosmetics industry, the use of transdermal medications is quite high. It is easier for people to apply topical solutions to their skin wherever required, when advised to do so. It is very important to evaluate the active ingredients in these transdermal drugs, and that is where the role of Franz cells and vapometers comes into play.
These Franz cell systems are designed to imitate the behavior of actives and formulations when applied to the skin. This increase in demand represents the growth of the Franz cell and vapometer market.
The skin's physiology acts as a barrier to the optimized delivery and permeation of transdermal drugs. These barriers are characterized as mechanical barriers; for example, the stratum corneum, tight and junctions. This stratum corneum consists of dead keratinocytes and lipids, forming a molecular architecture that drug molecules must permeate to be effective. The barrier function of the skin is calculated using the transepidermal water loss measurement device, such as a vapometer.
Researchers focus on developing drugs that are successful. These strategies are none other than enhancement methods used for increasing the penetration and permeation capabilities of transdermal drug products. Enhancement methods include vesicles and particles, energy-driven methods, stratum corneum modification, and drug-vehicle interactions. These barriers hinder the development of transdermal drugs.
The growing use of advanced membrane technologies in dermatology and transdermal research is creating a strong opportunity for customized Franz cell systems tailored for novel materials. As labs increasingly adopt synthetic skin models, 3D-bioprinted tissues, reconstructed epidermis, and polymeric barrier films, traditional diffusion cells often fail to provide optimal fit, sealing, or physiological relevance. Manufacturers can innovate by developing adaptable donor–receptor interfaces, flexible clamping mechanisms, and variable diffusion surface areas to accommodate these new substrates. Such customized systems will enable more accurate permeation studies, support cutting-edge formulation testing, and accelerate the development of next-generation topical drugs, patches, and skincare technologies.
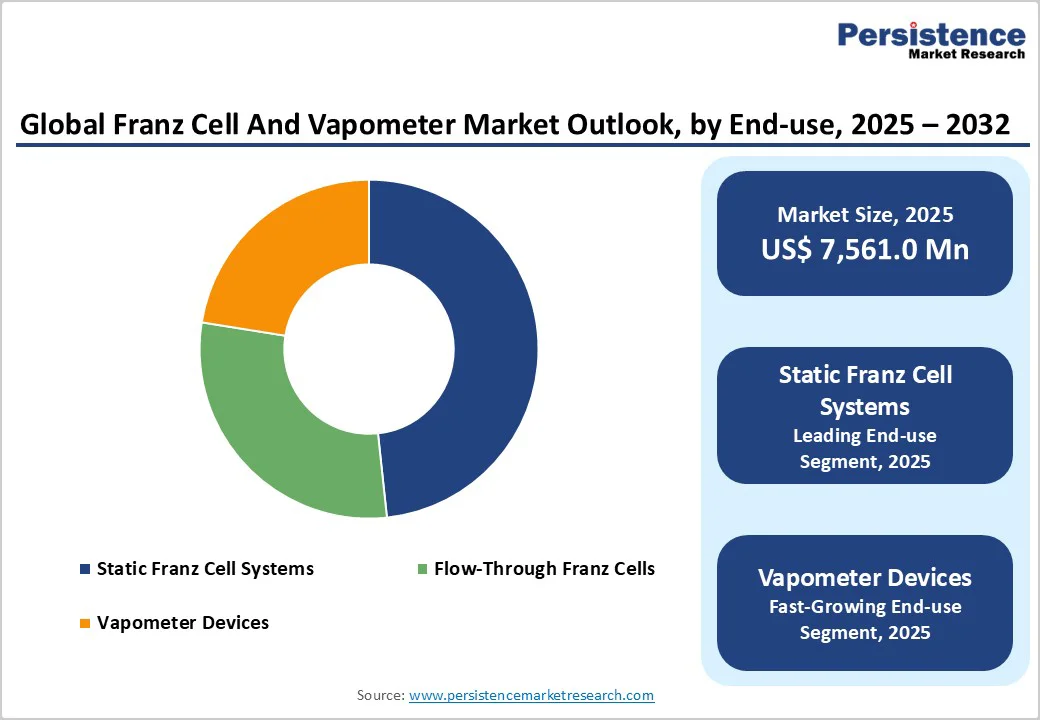
Static Franz Cell Systems will hold the highest market share because they remain the industry’s most trusted and widely standardized platform for in-vitro permeation testing. Their simple design, low cost, and minimal technical requirements make them accessible to pharmaceutical companies, cosmetic formulators, CROs, and academic labs worldwide. Regulatory guidelines for IVPT and generic topical bioequivalence predominantly reference static systems, reinforcing their widespread adoption. Additionally, their versatility across diverse formulations, creams, gels, ointments, nanoparticles, and transdermal patches ensures consistent demand. With strong reproducibility, broad manufacturer availability, and lower operational complexity than flow-through systems, static Franz cells naturally lead overall market penetration.
Pharmaceutical and biotechnology companies hold the highest share in the Franz Cell and Vapometer market because these tools are essential for developing topical, transdermal, and dermatology drug products. Pharma companies routinely perform in vitro permeation testing (IVPT), release studies, and bioequivalence assessments to meet stringent regulatory requirements for new formulations and generic approvals.
Their extensive R&D pipelines, high testing volumes, and continuous need for validated diffusion and TEWL data drive consistent demand. Additionally, pharma invests heavily in advanced, automated Franz cell systems to accelerate formulation optimization. This strong dependence on permeation science makes pharma the dominant end-user segment in the market.
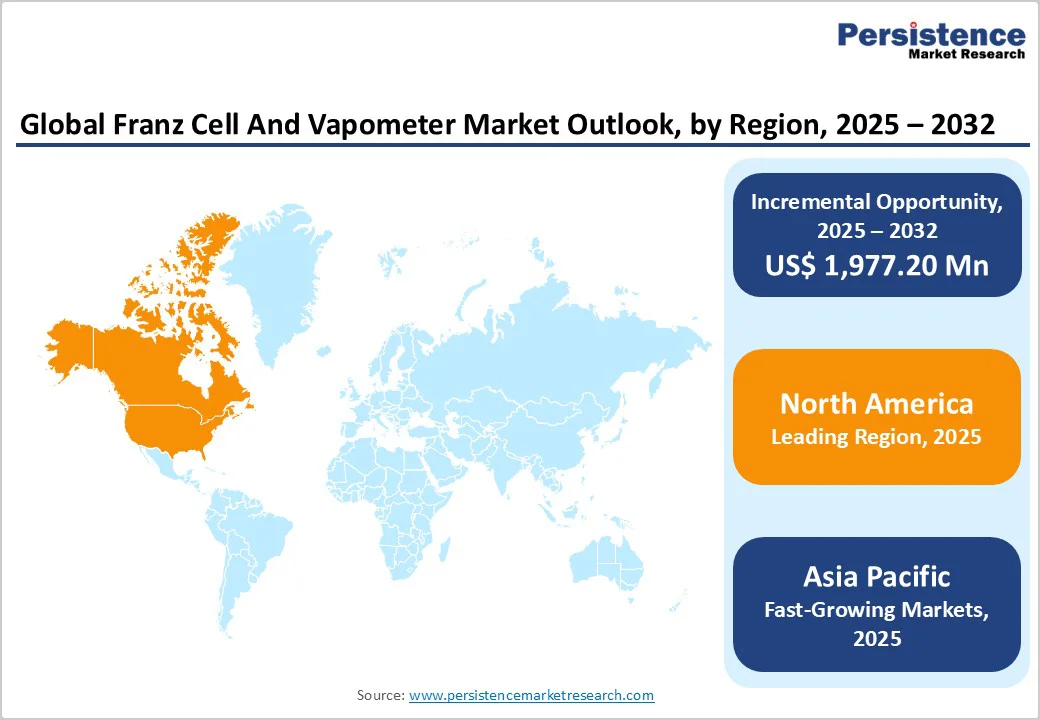
North America leads the Franz Cell and Vapometer market due to its strong pharmaceutical research ecosystem, high regulatory standards, and widespread adoption of topical and transdermal drug development. The U.S. dominates regional demand, supported by extensive FDA-driven IVPT requirements, advanced dermatology research, and the presence of major diffusion system manufacturers. Continuous investment in automated Franz cells, TEWL-based skin barrier analysis, and formulation innovation further strengthens the region’s leadership. The growing development of generic topicals and biosimilar dermatology products also drives consistent use of permeation and water-loss measurement tools across U.S. pharmaceutical companies, CROs, and academic research centers.
Asia Pacific is witnessing strong growth in the Franz Cell and Vapometer market, driven by expanding pharmaceutical manufacturing, rising dermatology research, and increasing development of transdermal and topical formulations. China and India are investing heavily in in-vitro permeation testing capabilities, while Japan and South Korea lead in advanced skincare and TEWL-based barrier studies.
Growing generic drug production, increasing CRO activities, and higher adoption of automated diffusion systems are accelerating market demand. Additionally, regulatory modernization and the rise of specialized skin models and bioprinted tissues are encouraging wider use of Franz cells and vapometers across academic institutes, formulation labs, and emerging biotech companies in the region.
The Franz Cell and Vapometer market is moderately competitive, with manufacturers focusing on accuracy, reproducibility, and user-friendly designs. Companies compete through innovations in automated sampling, improved temperature control, and high-throughput diffusion systems. Vapometer device makers emphasize precision TEWL measurement and ergonomic, portable designs. Pricing, product reliability, regulatory compliance support, and strong regional distribution networks significantly influence purchasing decisions.
The global franz cell and vapometer market is projected to be valued at US$7,561.0 Mn in 2025.
Increasing R&D in creams, gels, ointments, and patches requires accurate in-vitro permeation testing using Franz cells.
The global franz cell and vapometer market is poised to witness a CAGR of 3.4% between 2025 and 2032.
Designing diffusion cells compatible with synthetic skin, 3D-bioprinted tissues, and advanced polymeric films.
Delfin Technologies, Biox Systems Ltd., PermeGear, Inc., Orchid Scientific & Innovative India Pvt Ltd., and others
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn, Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author