ID: PMRREP31877| 202 Pages | 19 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

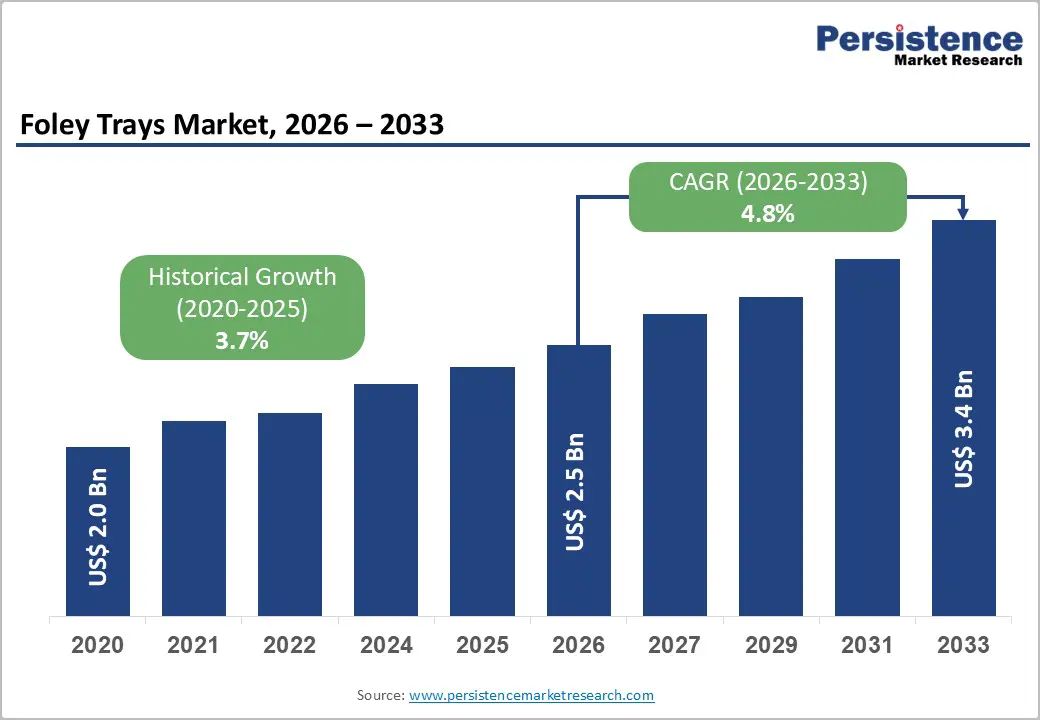
The global foley trays market size is likely to be valued at US$2.5 billion in 2026, and is expected to reach US$3.4 billion by 2033, growing at a CAGR of 4.8% during the forecast period from 2026 to 2033, driven by the increasing prevalence of urinary incontinence, rising demand for infection-resistant Foley catheter trays in hospitals, and advancements in hydrophilic-coated and silicone materials.
Growing demand for safe, ready-to-use foley trays, especially in long-term care and hospitals, is accelerating adoption across end-users. Advances in 3-way and suprapubic catheter trays are further boosting uptake by offering better drainage and reduced complications. Increasing recognition of Foley trays as critical for infection control and patient comfort in emerging healthcare markets remains a major driver of market growth.
| Key Insights | Details |
|---|---|
| Foley Trays Market Size (2026E) | US$2.5 Bn |
| Market Value Forecast (2033F) | US$3.4 Bn |
| Projected Growth (CAGR 2026 to 2033) | 4.8% |
| Historical Market Growth (CAGR 2020 to 2025) | 3.7% |
The rising prevalence of urinary incontinence is emerging as a major healthcare concern, driven largely by demographic, lifestyle, and medical factors. One of the primary contributors is the aging global population, as bladder control naturally declines with age and age-related conditions, such as benign prostatic hyperplasia, menopause-related changes, and neurological disorders, become more common. With increasing life expectancy, a greater proportion of individuals require long-term urinary management solutions. A study published in the Urology Journal (October 2024), titled “Efficacy and Safety of a Self-Improved Continuous Bladder Irrigation Sensor Device,” found that patients using the sensor-integrated system experienced a significantly shorter hospital stay of 3.3 days compared to 3.6 days for those receiving conventional manual care, underscoring the efficiency and safety advantages of such intelligent systems.
Lifestyle changes are also contributing to the rise in urinary incontinence. Increasing rates of obesity, sedentary behavior, and weakened pelvic muscles place additional pressure on the bladder, resulting in stress and urge incontinence among both elderly and middle-aged populations. In women, pregnancy, childbirth, and postpartum complications markedly increase risk, while in men, prostate surgeries and treatments can lead to temporary or permanent incontinence. The growing prevalence of chronic conditions, such as diabetes, stroke, Parkinson’s disease, and spinal injuries, has expanded the population affected by bladder dysfunction. Enhanced awareness and improved diagnostic practices have further increased reported cases, as more individuals seek medical care instead of dismissing symptoms as a normal part of aging.
High development and regulatory costs pose a major barrier for companies working on next-generation Foley trays and innovative catheter systems. Creating advanced designs, such as pre-connected hydrophilic silicone, low-trauma coude, or antimicrobial 3-way trays, requires extensive research, biocompatibility testing, and specialized coating technologies, all of which are significantly more expensive than standard latex trays. Safety adds another layer of complexity: refined variants, infection-resistant lots, and long-dwell products are highly sensitive to encrustation, biofilm formation, and tissue irritation, necessitating rigorous optimization to ensure safe performance throughout indwelling. Achieving consistent long-term functionality often involves costly clinical trials, advanced microbiological testing, and the use of premium polymers, driving R&D expenditures even higher.
Compliance with strict regulatory requirements for sterility, CAUTI rates, and batch consistency demands multiple validation studies across diverse conditions and patient groups, adding both time and cost to development. Scaling up manufacturing further increases expenses, requiring controlled cleanrooms, specialized assembly lines, and robust quality assurance systems. For smaller manufacturers, these financial and operational challenges can constrain innovation or delay product commercialization.
Innovations in hydrophilic-coated and antimicrobial Foley tray delivery systems are reshaping the global urology market by addressing two key challenges: infection prevention and insertion discomfort. Hydrophilic platforms are designed to provide ultra-low friction, reducing the need for additional lubricants and enabling smoother, more comfortable catheterization. Advanced features, such as pre-hydrated coatings, integrated drainage bags, antimicrobial silver ions, and anti-encrustation technologies, enhance patient comfort while lowering CAUTI rates, ultimately reducing hospital costs and improving patient outcomes.
Advances in antimicrobial platforms, including silicone elastomer-coated latex, noble metal alloys, antibiotic-impregnated materials, and biofilm-resistant designs, help ensure safer indwelling by minimizing bacterial adhesion, the first line of defense against complications. These solutions reduce irritation, extend dwell time, and support versatile use without frequent changes, making them ideal for large-scale long-term care programs. Emerging technologies, such as bio-adhesive coatings, VLP-based antimicrobials, and AI-monitored drainage systems, further enhance safety and responsiveness.
Straight catheters are anticipated to dominate the market, accounting for approximately 55% of the market share in 2026. Its dominance is driven by simplicity, wide applicability, and cost-effectiveness, making it preferred for routine indwelling. Straight catheters provide reliable drainage, ensure ease of insertion, and contribute to volume, making them suitable for large-scale hospital campaigns. Becton, Dickinson and Company (BD) is a leading global medical technology company whose urology product lines include a broad range of intermittent/straight catheters designed for routine bladder drainage in hospitals, long-term care, and home care settings. BD’s catheter portfolio features simple, reliable designs that enable easy insertion and dependable urine drainage, making them a common choice for clinicians managing urinary retention, incontinence, and postoperative care.
3-Way and hematuria catheter represents the fastest-growing segment, due to its irrigation capability and expanding use in post-surgical and hematuria cases. Its continuous irrigation profile makes it ideal for targeted bladder management, reducing clot formation. Continuous innovations in design are further strengthening its acceptance, driving rapid adoption across North America and Europe, where demand for specialized urology is accelerating. UNOQUIP GmbH, a Switzerland-based medical device manufacturer, launched a new range of postoperative urological catheters, including a Hematuria Management Catheter and a 3-Way Silicone Foley Catheter designed specifically for continuous bladder irrigation following urological surgeries.
The silicone segment is expected to lead the market, holding approximately 35% of the share in 2026, driven by superior biocompatibility, long-term dwell, and reduced irritation, making it preferred for extended use. The dominance continues as guidelines favor silicone over latex. Rising adoption of hydrophilic-coated latex and expanded Teflon campaigns highlights the growing focus on comfort alternatives. Rusch Brillant - Silicone Foley Catheter from Teleflex Incorporated is a widely used 100% silicone Foley catheter designed for indwelling urinary catheterization, particularly when long-term placement and high biocompatibility are required. Silicone as a material offers excellent biocompatibility and reduced tissue irritation, making it preferable for extended dwell times compared with traditional latex catheters, a major driver of adoption in hospitals and chronic care settings.
Hydrophilic polymer-coated latex is likely to be the fastest-growing segment, due to strong momentum in low-friction insertion and expanding inclusion in pain-sensitive patients. The growing shift toward comfort platforms, along with better acceptance, accelerates the adoption. Advancements in coating durability and the continued progress of pre-lubricated entering clinical trials drive market growth. AMSure® Hydrophilic Coated Latex Foley Catheters utilize a proprietary coating system to increase lubricity, helping to improve patient comfort. The foley catheters are 11 in. and 16 in long, available in a variety of French and balloon sizes.
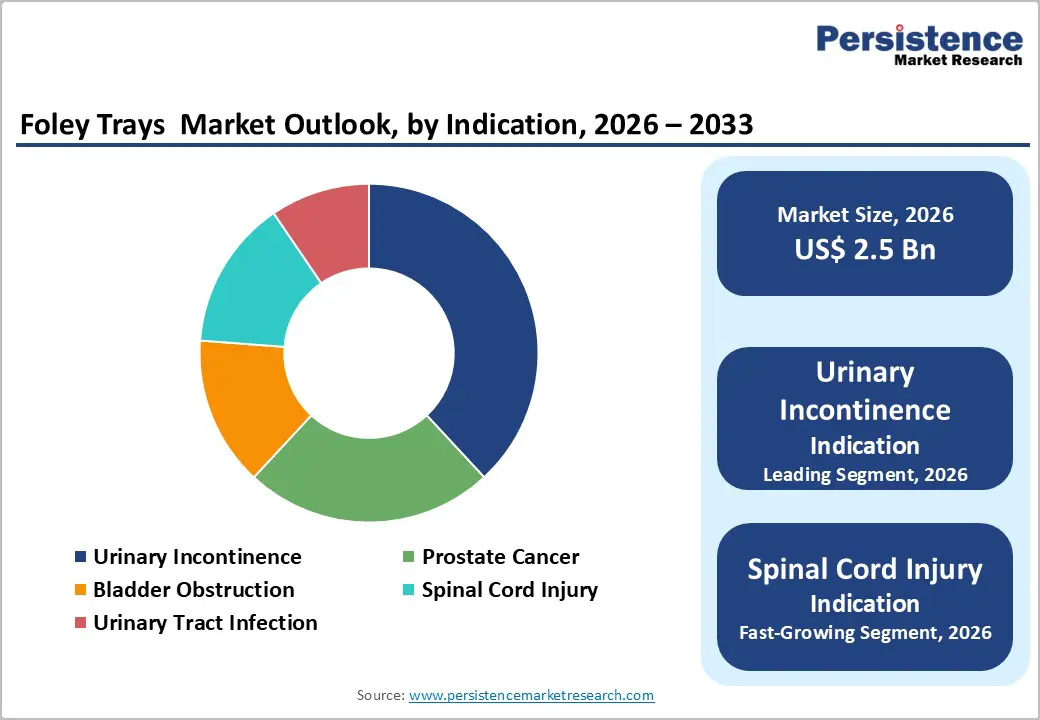
The urinary incontinence segment is projected to lead the market, accounting for nearly 40% of revenue in 2026. This is driven by its continued role as the primary indication for chronic indwelling catheters, extensive elderly care programs, and the management of patients requiring long-term catheterization. High integration, trained urologists, and the ability to manage high-volume or long-dwell applications contribute to increased usage. The incontinence sector is also spearheading the adoption of silicone catheters and conducting emerging trials with hydrophilic products. Coloplast A/S, a global leader in urology solutions, offers products such as SpeediCath® and Self-Cath™, specifically designed for urinary incontinence and chronic bladder management. These catheters are widely utilized across long-term care facilities, home care, and hospitals to manage involuntary bladder emptying in elderly patients and those with chronic neurological conditions.
The spinal cord injury segment is likely the fastest-growing market, driven by the prevalence of neurogenic bladder and the expanding role of rehabilitation programs. Catheter solutions in this segment provide convenient, quick, and accessible drainage, appealing to caregivers seeking reliable, low-complication options. Enhanced outreach initiatives, a focus on neurological care, and greater availability of both routine and premium catheter trays further accelerate adoption, particularly across urban and semi-urban areas. Coloplast A/S has launched the SpeediCath® Flex Set intermittent catheter, designed to enhance the catheterization experience for patients with neurogenic bladder and spinal cord injuries.
Hospitals are expected to remain the dominant end-user segment, accounting for over 45% of the market share in 2026. They continue to serve as primary centers for surgical procedures, emergency care, and long-term patient management. High patient volumes, access to trained clinicians, and advanced infrastructure support extensive catheter usage. Hospitals also manage complex cases requiring continuous monitoring, extended catheterization, and post-operative care, leading to consistently higher product consumption compared to other settings.
Ambulatory surgical centers (ASCs) are projected to be the fastest-growing segment between 2026 and 2033, driven by the increasing shift toward outpatient and minimally invasive procedures. These centers enable quicker patient turnover, lower treatment costs, and shorter hospital stays, thereby increasing procedural volumes. Rising numbers of urology, orthopedic, and day-care surgeries further boost catheter usage. Enhanced investments, improved infrastructure, and patient preference for same-day discharge are accelerating adoption in ASCs. United Surgical Partners International (USPI), one of the largest ASC operators in the U.S., manages over 400 outpatient surgery facilities offering a wide range of procedures, including urology, orthopedics, ophthalmology, and minimally invasive treatments. Through partnerships with physician groups and health systems, USPI has expanded its ASC network, facilitating higher outpatient surgical volumes and same-day care options that traditionally required hospital stays.
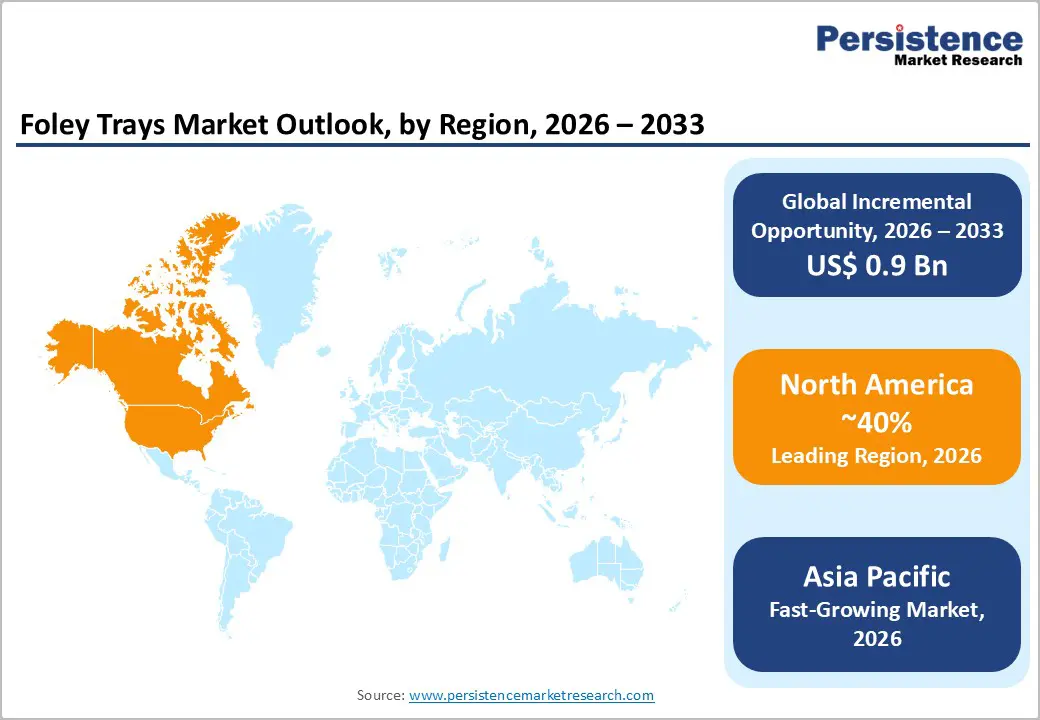
North America is projected to lead the Foley trays market, capturing nearly 40% of the share by 2026, driven by the region’s advanced urology infrastructure, robust research and development capabilities, and high public awareness of infection prevention benefits. Healthcare systems in the U.S. and Canada offer extensive support for catheterization programs, ensuring broad access to Foley trays across hospitals, long-term care facilities, and clinics. Growing demand for silicone-based, convenient, and user-friendly designs is further accelerating adoption, as these options enhance patient comfort and reduce barriers associated with latex products.
Technological advancements in Foley trays, such as stable hydrophilic coatings, enhanced silicone delivery, and infection-targeted innovations, are attracting substantial investment from both public and private sectors. Government initiatives and CMS campaigns continue to encourage their use to mitigate CAUTI risks, long-term complications, and emerging urological challenges, sustaining market demand. The increasing focus on 3-way catheters and specialty applications, particularly for spinal cord injury patients, is broadening the scope of Foley tray usage.
Europe is supported by increasing awareness of infection benefits, strong healthcare systems, and government-led quality programs. Countries such as Germany, France, and the U.K. have well-established urology frameworks that support routine Foley use and encourage adoption of innovative tray delivery methods, including Foley trays. These safe formulations are particularly appealing for incontinence populations, regulation-conscious operators, and long-term care users, improving compliance and coverage rates.
Technological advancements in foley trays, such as enhanced silicone, application-targeted delivery, and improved hydrophilic grades, are further boosting market potential. European authorities are increasingly supporting research and trials for trays against both routine and specialized needs, strengthening market confidence. The growing emphasis on convenient, low-risk options is aligned with the region’s focus on preventive urology and reducing hospital-acquired infections. Public awareness campaigns and promotion drives are expanding reach in both urban and rural areas, while suppliers are investing in coating and novel variants to increase efficacy.
Asia Pacific is likely to be the fastest-growing market for foley trays in 2026, driven by rising urological awareness, increasing government initiatives, and expanding application programs across the region. Countries such as India, China, Japan, and Southeast Asian nations are actively promoting tray campaigns to address aging growth and emerging hospital needs. Foley trays are particularly attractive in these regions due to their cost-effective administration, ease of scaling, and suitability for large-scale care drives in both urban and rural populations.
Technological advancements are supporting the development of stable, effective, and easy-to-use Foley trays, which can withstand challenging access conditions and minimize infection risk. These innovations are critical for reaching remote facilities and improving overall catheter coverage. Growing demand for incontinence, prostate cancer, and bladder obstruction applications is contributing to market expansion. Public-private partnerships, increased healthcare expenditure, and rising investment in urology research and manufacturing capacity are further accelerating growth. The convenience of tray delivery, combined with improved comfort and reduced risk of CAUTI, positions Foley trays as a preferred choice.
The global foley trays market features competition between established medical device leaders and emerging urology specialists. In North America and Europe, Teleflex Incorporated and Medline lead through strong R&D, distribution networks, and hospital ties, bolstered by innovative silicone and hydrophilic programs. In Asia Pacific, local manufacturers advance with cost-competitive solutions, enhancing accessibility. Silicone delivery boosts biocompatibility, cuts CAUTI risks, and enables mass integrations across regions. Strategic partnerships, collaborations, and acquisitions merge expertise, expand portfolios, and speed commercialization. Hydrophilic formulations solve comfort issues, aiding penetration in long-term areas.
The global foley trays market is projected to reach US$2.5 billion in 2026.
The foley trays market is primarily driven by the increasing incidence of urinary incontinence and the growing demand for foley catheter trays that are resistant to infections.
The foley trays market is poised to witness a CAGR of 4.8% from 2026 to 2033.
Significant market opportunities lie in the development of hydrophilic-coated and antimicrobial delivery systems.
Teleflex Incorporated, Medline, Becton, Dickinson and Company (BD), Cardinal Health, and Coloplast are the key players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020-2025 |
| Forecast Period | 2026-2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Catheter Type
By Material
By Indication
By End-users
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author