ID: PMRREP28738| 191 Pages | 9 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

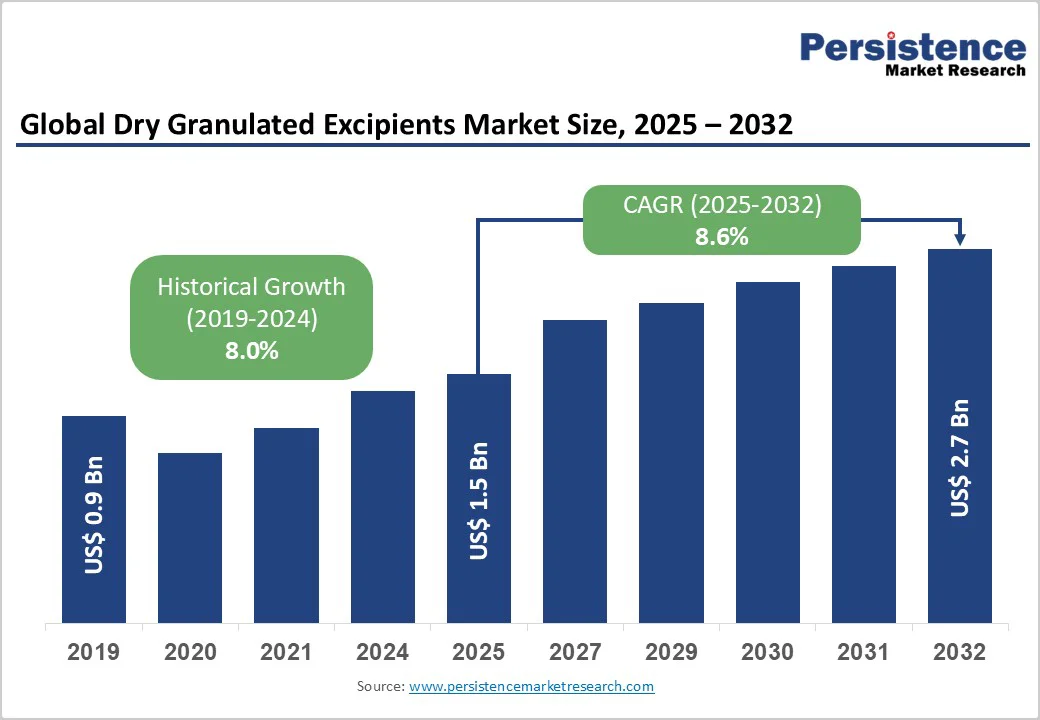
The global dry granulated excipients market size is likely to be valued US$1.5 Billion in 2025, growing to US$2.7 Billion by 2032 at a CAGR of 8.6% during the forecast period from 2025 to 2032 driven by the increasing demand for efficient pharmaceutical formulations, rising adoption of dry granulation techniques in drug manufacturing, and advancements in excipient technologies for improved flowability and compressibility.
The market is further propelled by innovations in multifunctional excipients and bio-based formulations, catering to preferences for sustainable and high-performance materials.
| Key Insights | Details |
|---|---|
|
Dry Granulated Excipients Market Size (2025E) |
US$1.5 Bn |
|
Market Value Forecast (2032F) |
US$2.7 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
8.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.0% |
The increasing demand for efficient pharmaceutical formulations and the adoption of dry granulation techniques are primary drivers of the dry granulated excipients market. Oral solid dosage (OSD) forms dominate pharmaceutical production, and dry granulation is particularly suitable for moisture-sensitive APIs, enhancing powder flow, compressibility, and overall manufacturing efficiency. Companies such as DFE Pharma have developed excipients that significantly reduce processing time in roll compaction, supporting cost-effective production compared to traditional wet granulation methods.
The growth of generic drugs and contract manufacturing has further accelerated the adoption of dry granulated excipients, as manufacturers seek scalable, reliable, and faster production processes. Additionally, the industry’s increasing focus on sustainable and eco-friendly manufacturing, in line with regulatory guidelines, is boosting demand for bio-based and multifunctional excipients. Technological advancements, including modular formulations and integration with emerging methods such as 3D printing, allow for greater customization and improved drug performance.
Higher production costs and performance limitations of dry granulated excipients pose significant restraints on market growth. While they are effective for moisture-sensitive APIs and enable efficient direct compression, specialized excipients often require advanced processing techniques, such as roll compaction, which can increase formulation complexity and overall manufacturing costs, making adoption challenging in cost-sensitive markets.
Performance-related issues, such as inconsistent tablet hardness or reduced disintegration efficiency with some binders, may necessitate additional optimization, extend development timelines, and increase resource requirements. Regulatory compliance for novel excipients adds another layer of complexity, as thorough testing and documentation are needed to meet safety and quality standards, which can delay product launches.
Advancements in bio-based and multifunctional excipients present significant growth opportunities for the dry granulated excipients market. Multifunctional designs enable simultaneous binding and disintegration, enhancing tablet performance and formulation efficiency, while supporting controlled drug release and precise delivery for complex biologics. Companies such as Colorcon are investing in plant-derived and naturally sourced fillers, improving stability, and aligning with the growing demand for sustainable ingredients.
Modular excipient formulations help streamline development processes, reduce production complexity, and allow better customization of oral solid dosage forms. Integration with emerging technologies, such as 3D printing, further enables tailored drug delivery systems and personalized medicine applications. Additionally, rising consumer preference for eco-friendly and clean-label products is driving demand for bio-based excipients across pharmaceuticals, nutraceuticals, and functional supplements.
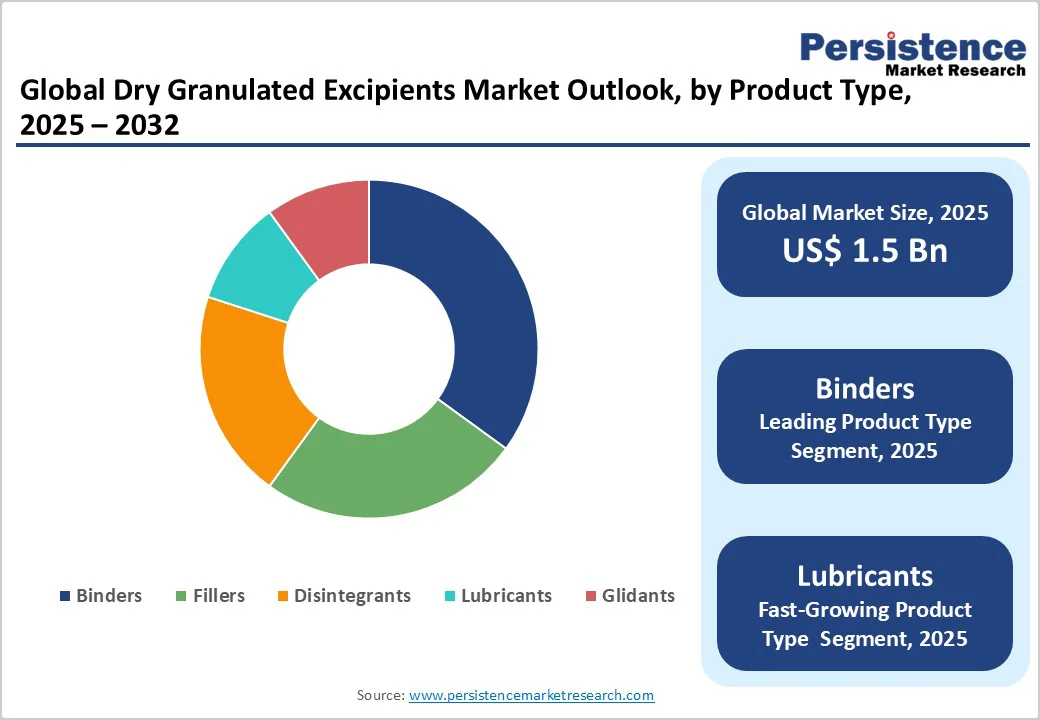
Binders dominate the market, account 40% market share in 2025. Their prominence stems from their essential role in particle aggregation and enhancing tablet strength, ensuring uniformity and stability in oral solid dosage forms. This makes binders indispensable in dry granulation processes, supporting efficient manufacturing and high-quality pharmaceutical production.
Lubricants are the fastest-growing segment, driven by increasing demand for improved powder flow, reduced friction, and enhanced tablet ejection in high-speed tableting. Their use ensures consistent tablet quality, minimizes production issues, and supports efficiency in large-scale pharmaceutical manufacturing, leading to rapid adoption across OSD production processes.
Compression leads with 35% share in 2025, due to its efficiency in direct compaction processes for oral solid dosage (OSD) forms. It reduces manufacturing steps, lowers production costs, and ensures uniform tablet weight and content. These advantages make compression the preferred choice for large-scale pharmaceutical production, balancing quality, speed, and economic feasibility.
Drug Release Control is the fastest-growing, driven by the rising demand for sustained-release and controlled-release formulations, especially for chronic disease treatments. These excipients enable precise modulation of drug release, improving therapeutic efficacy, patient compliance, and dosage convenience, while supporting the development of advanced oral and specialized delivery systems in modern pharmaceuticals.
Pharmaceutical holds 60% share in 2025. These excipients are widely used to develop stable, high-performance drug delivery systems, enhancing formulation efficiency, bioavailability, and patient compliance. Their versatility allows manufacturers to create tablets, capsules, and other dosage forms with consistent quality, supporting both large-scale production and specialized therapeutic needs.
Nutraceutical is the fastest-growing, driven by increasing consumer demand for functional supplements and clean-label products. Manufacturers leverage these excipients to enhance stability, texture, and bioavailability in vitamins, minerals, and herbal formulations. Their versatility supports innovation in health-focused products, meeting regulatory standards and evolving preferences for natural, high-quality ingredients.
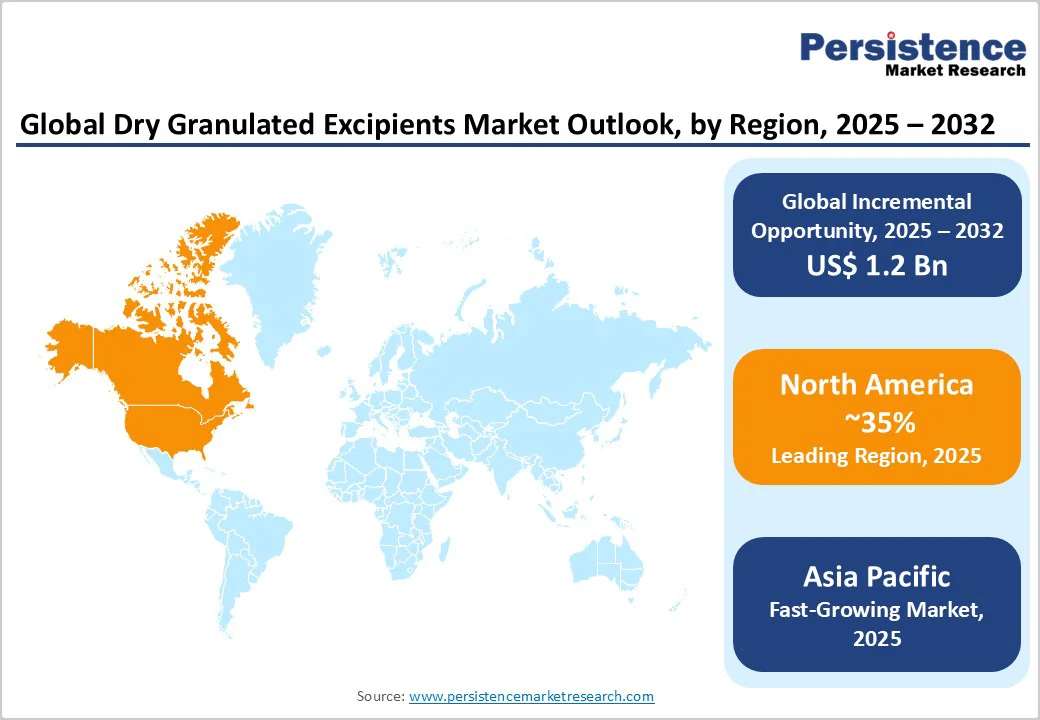
North America accounts for 35% in 2025, driven by advanced pharmaceutical research and development activities and a strong regulatory focus on efficient manufacturing processes. In the U.S. and Canada, the Food and Drug Administration (FDA) emphasizes quality, safety, and process efficiency, encouraging manufacturers to adopt high-performance, multifunctional excipients that streamline production and enhance drug formulation performance.
The region’s well-established pharmaceutical infrastructure, combined with significant investment in innovation, positions North America as a leader in developing excipients that meet complex formulation requirements, including sustained-release, taste-masking, and bioavailability-enhancing solutions.
Trends in multifunctional and bio-based excipients are particularly strong, as companies seek to optimize manufacturing efficiency while addressing regulatory and environmental expectations. Although the U.K. is part of Europe, it exhibits similar market dynamics, with increasing adoption of advanced excipients driven by the Medicines and Healthcare products Regulatory Agency (MHRA) guidelines and rising investments in the biopharma sector.
Europe holds about 30% market share, led by Germany and France. The region’s market leadership is largely influenced by stringent regulatory frameworks established by the European Medicines Agency (EMA), which emphasize quality, safety, and compliance in pharmaceutical manufacturing. These regulations drive the adoption of high-performance excipients that meet rigorous standards, ensuring consistency and efficacy in drug formulations.
A significant trend shaping the European market is the growing emphasis on sustainability. Pharmaceutical companies are increasingly prioritizing bio-based and environmentally friendly excipients to align with both regulatory expectations and evolving consumer preferences. This focus has prompted key players to invest in research and development of multifunctional, sustainable excipients that not only enhance drug performance but also reduce environmental impact.
Asia Pacific commands around 20% share and is the fastest-growing region, driven by the rapid expansion of the pharmaceutical sector in countries such as China and India. Both nations have emerged as major hubs for pharmaceutical manufacturing and contract development, attracting global attention due to their cost-efficient production capabilities and skilled workforce. The growth of contract manufacturing organizations (CMOs) in the region has further fueled demand for excipients, as these companies require reliable, high-quality ingredients to support large-scale production for both domestic and international markets.
Additionally, increasing investments in research and development, particularly in bio-based and multifunctional excipients, are helping local players enhance product offerings and compete with established Western manufacturers. Regulatory reforms and government initiatives to strengthen the pharmaceutical supply chain also contribute to market expansion. Furthermore, the region’s focus on cost-effective solutions allows Asia Pacific manufacturers to serve emerging and established markets efficiently.
The global dry granulated excipients market is highly competitive, featuring global giants and regional innovators. In North America and Europe, established companies such as DFE Pharma and BASF dominate the landscape, leveraging advanced research and development capabilities to produce multifunctional excipients that meet the evolving needs of pharmaceutical manufacturers. These regions focus heavily on innovation, with companies investing in bio-based and sustainable excipient solutions to align with regulatory standards and the growing preference for environmentally friendly products.
The Asia Pacific market is largely shaped by local and regional players who offer cost-effective excipient solutions, making the region attractive for manufacturers seeking economical alternatives without compromising quality. Across all regions, the emphasis on bio-based technologies is intensifying competition, as companies strive to differentiate themselves through sustainable offerings. Key strategic approaches include significant R&D investments in bio-based excipients, acquisitions to expand product portfolios, and collaborations with pharmaceutical companies to provide customized solutions.
The global dry granulated excipients market is projected to reach US$ 1.5 Bn in 2025, driven by surging demand for efficient pharma formulations amid rising OSD production worldwide.
Dry granulation techniques enhance powder flow, compressibility, and tablet uniformity while being suitable for moisture-sensitive APIs, supporting faster, cost-effective, and scalable manufacturing processes
The market is poised to witness a CAGR of 8.6% from 2025 to 2032, fueled by advancements in multifunctional and bio-based excipients for biopharma applications.
Advancements in bio-based excipients offer key opportunities, enabling enhanced stability and controlled release in sustainable drug delivery systems.
DFE Pharma, Gattefosse, BASF, Colorcon, and GEA Group are key players, leading through innovations in multifunctional excipients and global distribution networks.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Function
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author