- Executive Summary
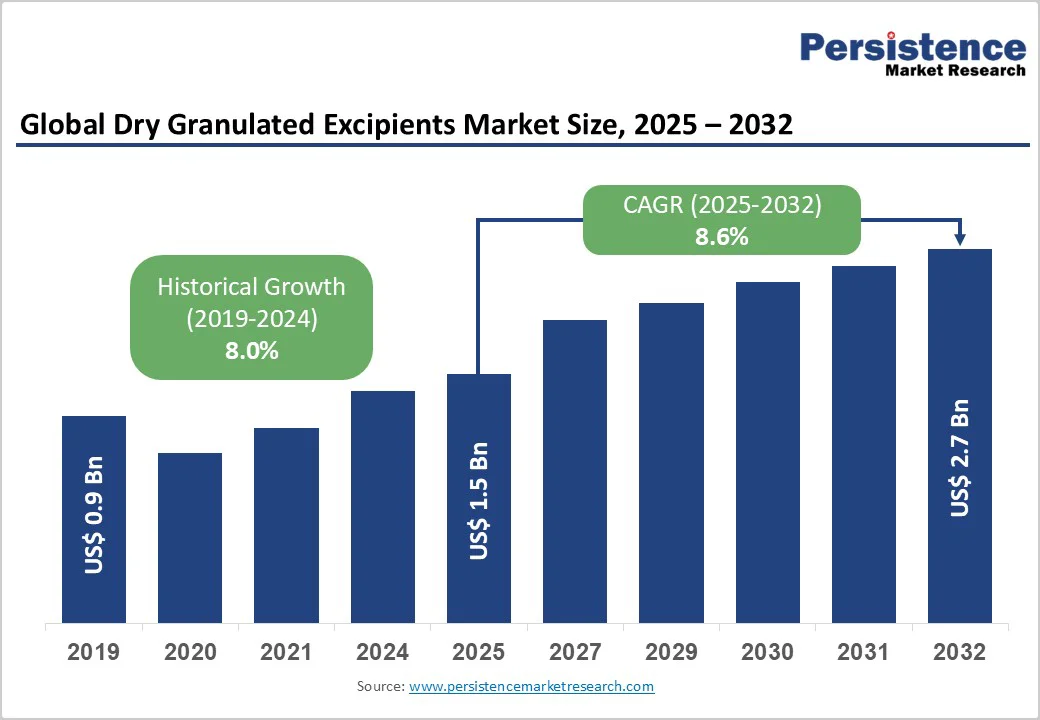
- Global Dry Granulated Excipients Market Snapshot, 2025 and 2032
- Market Opportunity Assessment, 2025 - 2032, US$ Bn
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments and Key Market Events
- PMR Analysis and Recommendations
- Market Overview
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Challenges
- Key Trends
- COVID-19 Impact Analysis
- Forecast Factors - Relevance and Impact
- Value Added Insights
- Value Chain Analysis
- Key Market Players
- Regulatory Landscape
- PESTLE Analysis
- Porter’s Five Force Analysis
- Consumer Behavior Analysis
- Price Trend Analysis, 2019 - 2032
- Key Factors Impacting Product Prices
- Pricing Analysis, By Product Type
- Regional Prices and Product Preferences
- Global Dry Granulated Excipients Market Outlook
- Market Size (US$ Bn) Analysis and Forecast
- Historical Market Size (US$ Bn) Analysis, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, 2025-2032
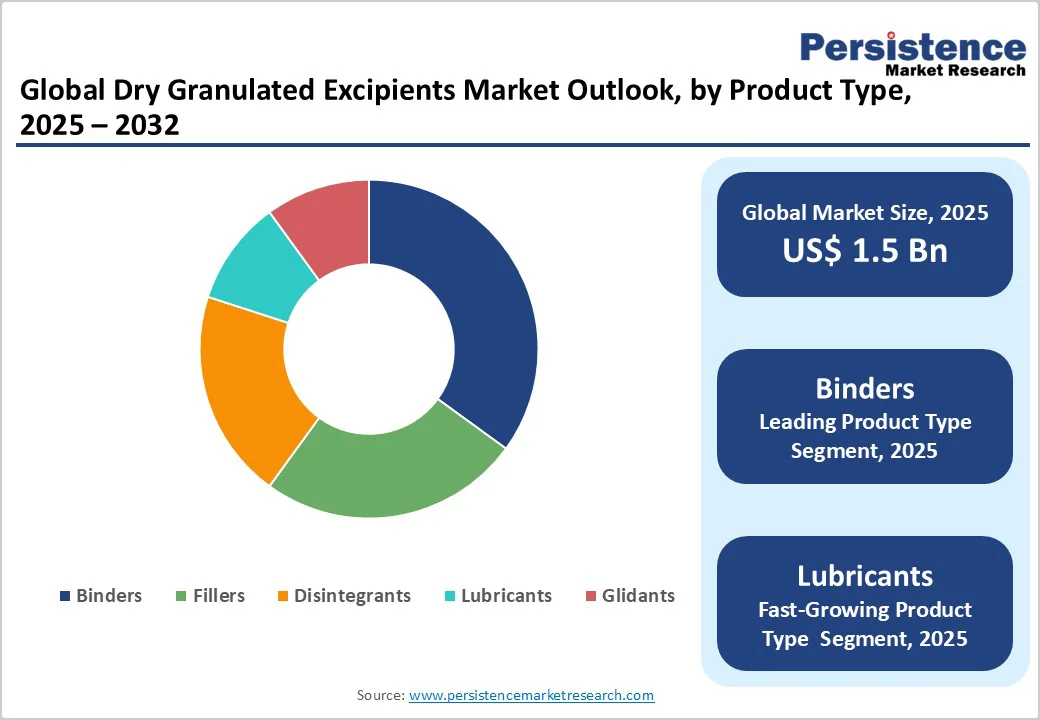
- Global Dry Granulated Excipients Market Outlook: Product Type
- Historical Market Size (US$ Bn) Analysis, By Product Type, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Attractiveness Analysis: Product Type
- Global Dry Granulated Excipients Market Outlook: Function
- Historical Market Size (US$ Bn) Analysis, By Function, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Attractiveness Analysis: Function
- Global Dry Granulated Excipients Market Outlook: Application
- Historical Market Size (US$ Bn) Analysis, By Application, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis: Application
- Market Size (US$ Bn) Analysis and Forecast
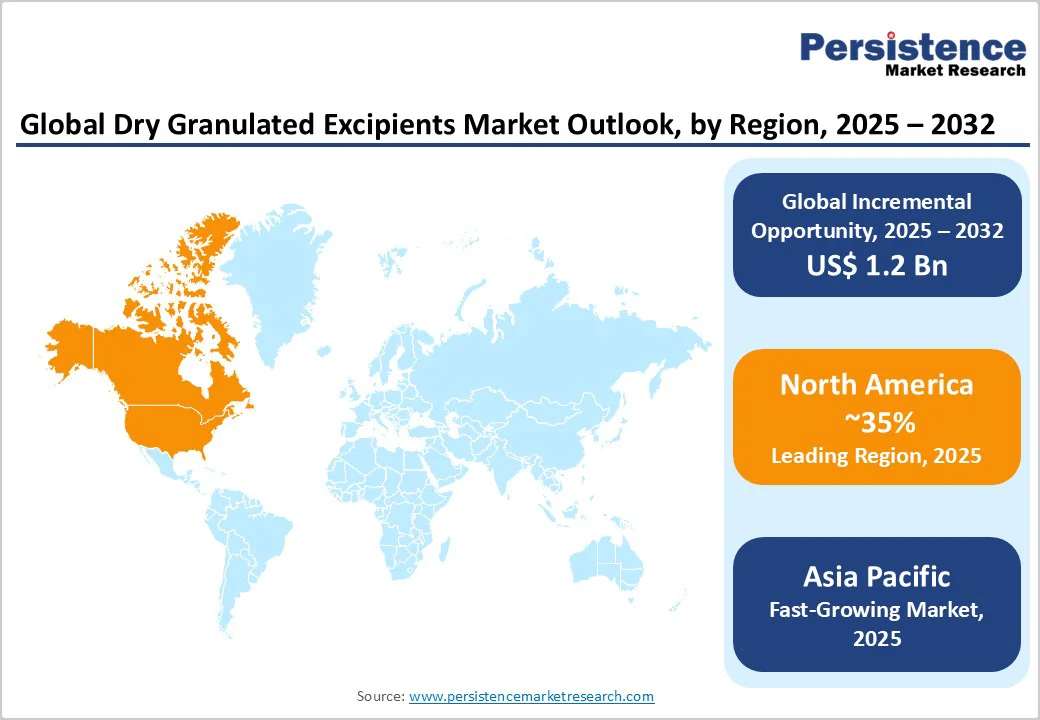
- Global Dry Granulated Excipients Market Outlook: Region
- Historical Market Size (US$ Bn) Analysis, By Region, 2019-2024
- Market Size (US$ Bn) Analysis and Forecast, By Region, 2025-2032
- North America
- Latin America
- Europe
- East Asia
- South Asia and Oceania
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- U.S.
- Canada
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Europe Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- Germany
- France
- U.K.
- Italy
- Spain
- Russia
- Rest of Europe
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- East Asia Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- China
- Japan
- South Korea
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- South Asia & Oceania Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- India
- Indonesia
- Thailand
- Singapore
- ANZ
- Rest of South Asia & Oceania
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Latin America Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- Brazil
- Mexico
- Rest of Latin America
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Middle East & Africa Dry Granulated Excipients Market Outlook
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- By Country
- By Product Type
- By Function
- By Application
- Market Size (US$ Bn) Analysis and Forecast, By Country, 2025-2032
- GCC Countries
- Egypt
- South Africa
- Northern Africa
- Rest of Middle East & Africa
- Market Size (US$ Bn) Analysis and Forecast, By Product Type, 2025-2032
- Binders
- Fillers
- Disintegrants
- Lubricants
- Glidants
- Market Size (US$ Bn) Analysis and Forecast, By Function, 2025-2032
- Compression
- Power Flowability
- Moisture Absorption
- Stability Enhancement
- Drug Release Control
- Market Size (US$ Bn) Analysis and Forecast, By Application, 2025-2032
- Pharmaceutical
- Nutraceutical
- Cosmetics
- Food and Beverages
- Market Attractiveness Analysis
- Historical Market Size (US$ Bn) Analysis, By Market, 2019-2024
- Competition Landscape
- Market Share Analysis, 2024
- Market Structure
- Competition Intensity Mapping By Market
- Competition Dashboard
- Company Profiles (Details - Overview, Financials, Strategy, Recent Developments)
- FE Pharma
- Overview
- Segments and Product Type
- Key Financials
- Market Developments
- Market Strategy
- Gattefosse
- BASF
- MultiMedia Pharma Sciences
- LLC
- Colorcon
- GEA Group Aktiengesellschaft
- Others
- FE Pharma
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment