ID: PMRREP28723| 200 Pages | 11 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

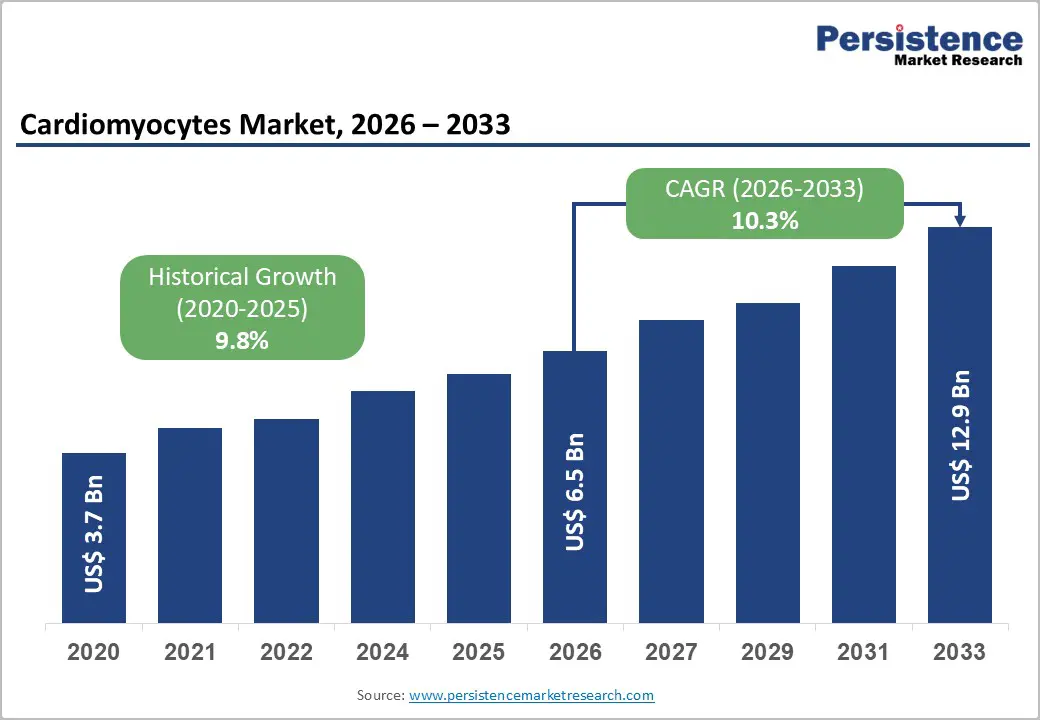
The global cardiomyocytes market size is likely to be valued at US$ 6.5 billion in 2026, and is projected to reach US$ 12.9 billion by 2033, growing at a CAGR of 10.3% during the forecast period 2026−2033. Market expansion reflects strong structural growth driven by rising cardiovascular disease prevalence, increasing pharmaceutical research and development spending, and rapid adoption of human-relevant in vitro models for drug discovery.
Cardiovascular diseases remain the leading cause of global mortality, reinforcing sustained demand for advanced cardiac research tools. Human-induced pluripotent stem cell-derived cardiomyocytes are increasingly replacing animal models due to their higher translational accuracy and growing regulatory acceptance in preclinical safety evaluation. Regulatory agencies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) promote predictive toxicology frameworks, further supporting market expansion.
| Key Insights | Details |
|---|---|
| Cardiomyocytes Market Size (2026E) | US$ 6.5 Bn |
| Market Value Forecast (2033F) | US$ 12.9 Bn |
| Projected Growth (CAGR 2026 to 2033) | 10.3% |
| Historical Market Growth (CAGR 2020 to 2025) | 9.8% |
Rising Prevalence of Cardiovascular Diseases
The prevalence of cardiovascular diseases has risen exponentially over the last decade, acting as a primary growth catalyst for cardiomyocyte adoption due to sustained pressure on healthcare systems to improve early-stage cardiac risk identification and therapeutic safety. According to World Health Organization (WHO) data, cardiovascular conditions remain the leading cause of death worldwide, highlighting the scale and persistence of cardiac disorders across both developed and emerging economies. This disease burden drives continuous expansion of cardiac drug pipelines, long-term safety testing, and disease modeling initiatives. Cardiomyocytes enable direct assessment of human cardiac responses at the cellular level, aligning closely with the clinical complexity of arrhythmia, heart failure, and ischemic conditions.
The underlying driver strength stems from structural changes in cardiovascular care and research priorities. Aging populations, lifestyle-related risk factors, and higher survival rates from acute cardiac events increase chronic disease management needs. This trend elevates demand for predictive platforms capable of evaluating cardiotoxicity, electrophysiology, and contractility with higher clinical relevance. Cardiomyocyte-based systems support precision medicine approaches, patient-specific modeling, and regenerative research, all aligned with evolving cardiology workflows. Pharmaceutical and biotechnology organizations integrate these models to reduce late-stage failures and optimize therapeutic profiles.
Limited Physiological Maturity and Multisystem Modeling
Constraints surrounding physiological maturity have diminished commercial and scientific value since in-vitro cardiomyocytes often lack adult-like sarcomere alignment, ion channel density, and mitochondrial efficiency required for reliable translational outcomes. Contractile force generation, calcium handling dynamics, and action potential duration frequently resemble neonatal profiles, creating distortion in dose–response relationships. Drug candidates targeting late-stage electrophysiological remodeling or chronic cardiac stress pathways face inconsistent signal detection.
Restricted multisystem modeling further limits applicability in complex therapeutic evaluation. Cardiovascular response is tightly linked to hepatic metabolism, renal clearance, endocrine signaling, and inflammatory modulation, none of which are captured in isolated cardiomyocyte platforms. Active metabolites generated outside cardiac tissue often drive toxicity profiles, yet remain unaccounted for during early screening. Precision medicine initiatives struggle to simulate patient-specific systemic variability using single-cell-type systems.
Expansion in Regenerative Medicine End-Users
Expansion in regenerative medicine End-Users represents a key opportunity as cardiomyocytes directly address unmet needs in cardiac repair and functional recovery following heart injury. Heart tissue shows limited self-regeneration capacity, creating strong demand for cell-based solutions that restore contractile function. Advances in stem cell differentiation, tissue engineering, and biofabrication enable scalable production of functional cardiac cells suitable for transplantation and tissue modeling. Growing investment in regenerative therapies aligns with long-term healthcare goals focused on reducing hospitalization rates, lowering lifetime treatment costs, and improving patient quality of life through durable therapeutic outcomes.
Clinical research momentum further strengthens this opportunity as cardiomyocyte-based approaches progress from experimental stages toward translational and clinical validation. For example, in November 2025, researchers from the University of Osaka conducted the first-in-human clinical application of allogeneic induced pluripotent stem cell-derived cardiomyocyte patches for treating non-ischemic dilated cardiomyopathy. The study demonstrated improved cardiac function and remuscularization without tumorigenesis or severe arrhythmias. Furthermore, integration with gene editing, biomaterials, and precision medicine strategies improves therapeutic targeting and safety profiles. Public and private funding flows increasingly prioritize regenerative platforms with clear commercialization potential, supporting infrastructure development and clinical trial expansion. Strategic partnerships between biotechnology firms, academic institutions, and healthcare providers accelerate validation pathways and shorten development timelines.
Product Type Insights
Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes are expected to capture approximately 65% of the global market share in 2026. Their widespread adoption is driven by their exceptional physiological similarity to human cardiac tissue, making them highly relevant for pharmaceutical, biotechnology, and academic research applications. These cells enable reliable cardiotoxicity testing, disease modelling, and drug discovery, meeting stringent regulatory standards. Scalable production methods, reproducible functional characteristics, and compatibility with high-throughput screening platforms significantly improve research efficiency and minimize risks in late-stage development. As a result, iPSC-derived cardiomyocytes are becoming the preferred choice for advancing cardiac research and therapeutic development.
Animal-derived cardiomyocytes are projected to experience the fastest growth between 2026 and 2033, primarily due to their ongoing use in legacy assay systems, comparative validation studies, and early-stage academic research. In some regions, regulatory guidelines still require animal-based preclinical testing, which continues to support demand for these cells. Their cost-effectiveness, well-established experimental protocols, and compatibility with historical datasets make them valuable for specific research workflows. Furthermore, technological advancements in cell isolation techniques, functional preservation, and handling efficiency are increasing their usability and expanding their role in cardiac research. These factors ensure that animal-derived cardiomyocytes will remain an essential resource alongside emerging human iPSC-based models.
Application Insights
Cardiac safety and toxicity testing is expected to account for nearly 48% of the cardiomyocytes market revenue share in 2026. This growth is driven by regulatory requirements that mandate thorough cardiotoxicity assessment for drugs targeting oncology, central nervous system, and metabolic disorders. The use of both human iPSC-derived and animal-derived cardiomyocytes has enabled high translational accuracy and reproducibility in preclinical safety evaluations. Standardized assay protocols, compatibility with high-throughput platforms, and consistent cell performance have reduced late-stage drug attrition and operational risk, making these approaches essential for modern drug development.
Cardiac disease modelling is projected to be the fastest-growing segment between 2026 and 2033. This expansion is supported by the increasing emphasis on precision medicine, patient-specific disease investigations, and research into genetic cardiomyopathies. Human iPSC-derived cardiomyocytes have proven effective in accurately replicating disease phenotypes, facilitating mechanistic studies, target validation, and drug discovery efforts. Contract research organizations are adopting independent assay workflows to provide reproducible, high-quality insights across various therapeutic areas. The integration of gene-editing tools, high-content imaging, and computational modelling further enhances experimental precision and predictive value, positioning disease modelling as a critical area for future cardiac research and therapeutic innovation.
End-User Insights
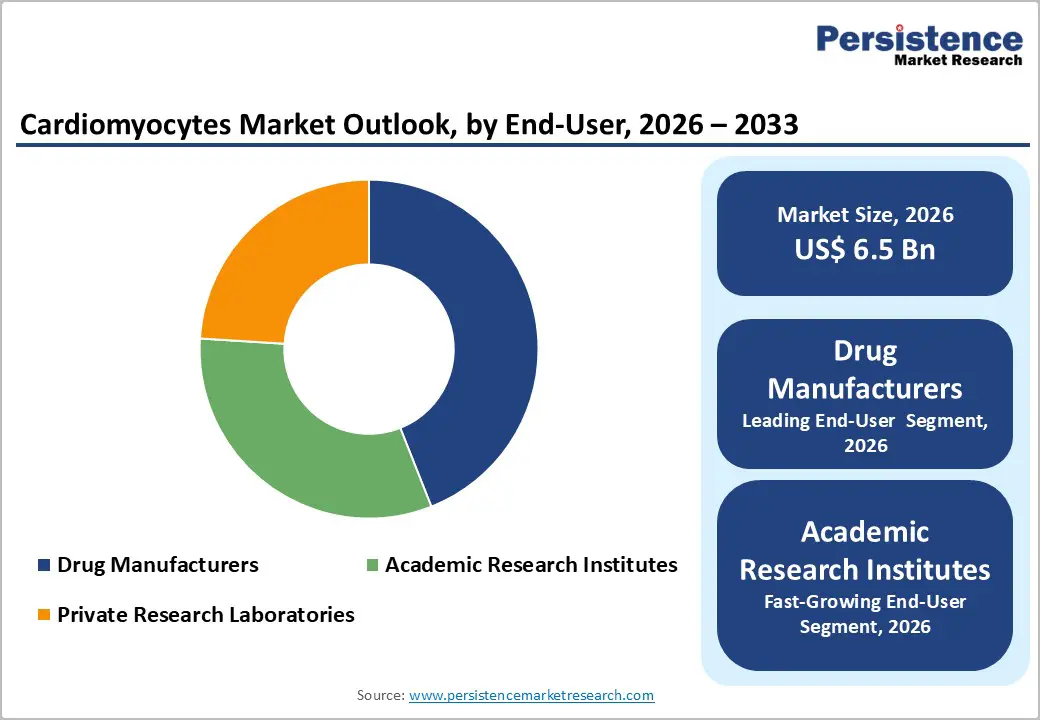
Drug manufacturers are expected to lead the end-user segment, capturing around 44% of the cardiomyocytes market share in 2026. Their broad adoption of cardiomyocyte platforms, such as human iPSC-derived and animal-derived cardiomyocytes, enables precise cardiotoxicity testing, disease modelling, and efficacy evaluation across oncology, cardiovascular, and central nervous system drug pipelines. Standardized protocols, alignment with regulatory requirements, and compatibility with high-throughput systems improve efficiency, minimize development risk, and support scalable testing for multiple therapeutic areas.
Academic research institutes are projected to be the fastest-growing end-user segment between 2026 and 2033. This growth is driven by increased government funding for translational cardiovascular research and a growing emphasis on patient-specific disease models and genetic cardiomyopathy studies. Collaborations with pharmaceutical companies and private research laboratories facilitate access to advanced cardiomyocyte platforms and standardized assay workflows. The integration of gene-editing technologies, high-content imaging, and high-throughput experimental systems further boosts research precision and reproducibility, positioning academic institutes at the forefront of cardiac research innovation.
North America Cardiomyocytes Market Trends
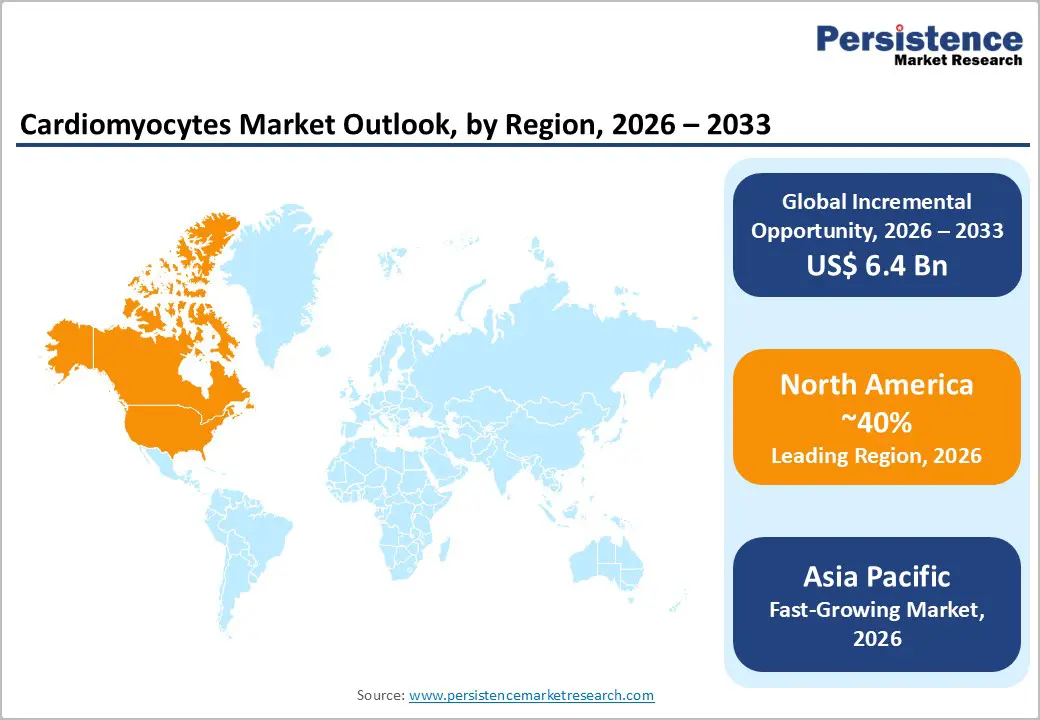
North America is projected to capture approximately 40% of the cardiomyocytes market share by 2026, driven by its advanced pharmaceutical and biotechnology ecosystem and early adoption of human iPSC-derived cardiomyocyte platforms. The region’s dominance is further strengthened by the presence of global pharmaceutical headquarters, leading contract research organizations, and academic research institutions with robust translational cardiovascular programs. Stringent regulatory frameworks, such as guidance from the U.S.FDA on predictive toxicology and cardiotoxicity testing, encourage the use of human-relevant in vitro models for drug development and safety assessment.
The regional market also benefits from state-of-the-art infrastructure supporting high-throughput screening, automated assay platforms, and scalable cell production, which significantly reduce time-to-data and enhance the predictability of translational outcomes. Close collaboration between academic institutions and industry players accelerates innovation in disease modelling, regenerative research, and personalized medicine. The availability of skilled research personnel, well-funded translational programs, and early adoption of advanced cell characterization and gene-editing techniques ensure consistent quality and reproducibility, reinforcing North America’s leadership in the market for cardiomyocytes.
Europe Cardiomyocytes Market Trends
Europe holds a significant position in the cardiomyocytes market, supported by advanced pharmaceutical infrastructure, strong research capabilities, and well-established regulatory frameworks that promote adoption of human-relevant in vitro cardiac models. The market is driven by a concentration of leading biotechnology and pharmaceutical companies in countries such as Germany, the United Kingdom, and France, which invest heavily in preclinical cardiac research and safety testing. Public funding and private investment initiatives support translational cardiovascular research, disease modelling, and regenerative medicine projects. Europe benefits from integration of cardiomyocytes into drug discovery pipelines, high-throughput screening, and personalized medicine programs, enhancing reproducibility and efficiency.
Strong collaborations between academic institutions, contract research organizations, and pharmaceutical firms accelerate innovation in disease-specific models and advanced cardiac assays. Regulatory guidance from the European Medicines Agency encourages the adoption of human iPSC-derived cardiomyocytes for cardiotoxicity and efficacy testing, further driving market utilization. Investment in advanced imaging, automation, and tissue engineering technologies strengthens research outcomes, while skilled scientific personnel and state-of-the-art laboratory facilities facilitate rapid adoption.
Asia Pacific Cardiomyocytes Market Trends
Asia Pacific is expected to be the fastest-growing market for cardiomyocytes between 2026 and 2033. This growth is fueled by the expansion of biotechnology hubs, increasing government support for regenerative medicine, and the rising adoption of advanced human iPSC-derived cardiac models in drug discovery and disease research. Rapid development of biotechnology centers in countries such as China, India, and Japan, coupled with growing domestic pharmaceutical research and development, is driving demand. Government-led initiatives and incentives for biotechnology startups are accelerating the use of human iPSC-derived cardiomyocytes and other in vitro platforms. Partnerships with multinational pharmaceutical companies and the establishment of regional centers for regenerative medicine are further strengthening research capabilities.
Cost advantages, a large pool of skilled life sciences professionals, and expanding laboratory infrastructure in the region enable large-scale experimentation and rapid deployment of cardiac research tools. The demand for disease-specific research and localized drug development targeting populations with high cardiovascular risk is also contributing to market growth. Investment in organ-on-chip systems, three-dimensional (3D) cardiac tissue models, high-throughput platforms, and advanced imaging technologies is broadening preclinical study capabilities. Collaboration between public institutions, private companies, and contract research organizations is fostering innovation in regenerative therapies and personalized medicine, positioning Asia Pacific as a key player in this market.
The global cardiomyocytes market structure is moderately fragmented. The top five companies – Thermo Fisher Scientific, FUJIFILM Cellular Dynamics, Lonza Group, Sartorius AG, and Merck KGaA – account for approximately 42% of global revenue. These leading firms are focused on developing standardized cardiomyocyte platforms, human iPSC-derived cell lines, and high-quality reagents to ensure reproducibility, functional consistency, and regulatory alignment for pharmaceutical research, regenerative medicine, and academic studies.
This moderate level of market fragmentation allows both established players and emerging manufacturers to innovate and meet diverse research needs. Key companies leverage their expertise in stem cell production, bioprocessing solutions, and advanced assay platforms, backed by strong research and development capabilities and extensive global distribution networks. Their emphasis on quality, technological innovation, and compliance with regulatory standards enables them to efficiently address growing demand for preclinical testing, disease modelling, and cardiac safety assessment across various sectors.
Key Industry Developments
The global cardiomyocytes market is projected to reach US$ 6.5 billion in 2026.
The market is driven by rising cardiovascular disease prevalence worldwide, heavy investments in R&D by pharmaceutical compnaies, and growing adoption of human-relevant in vitro models for drug discovery and safety testing.
The market is poised to witness a CAGR of 10.3% from 2026 to 2033.
Key market opportunities lie in regenerative medicine, precision cardiology, disease modelling, and advanced drug safety and efficacy testing using human iPSC-derived cardiomyocytes.
Some of the key market players include Thermo Fisher Scientific, FUJIFILM Cellular Dynamics, Lonza Group, Sartorius AG, and Merck KGaA.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By End-User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author